The Periodic Table of the Elements Learning Goals
The Periodic Table of the Elements

Learning Goals To be able to describe properties of the periodic table
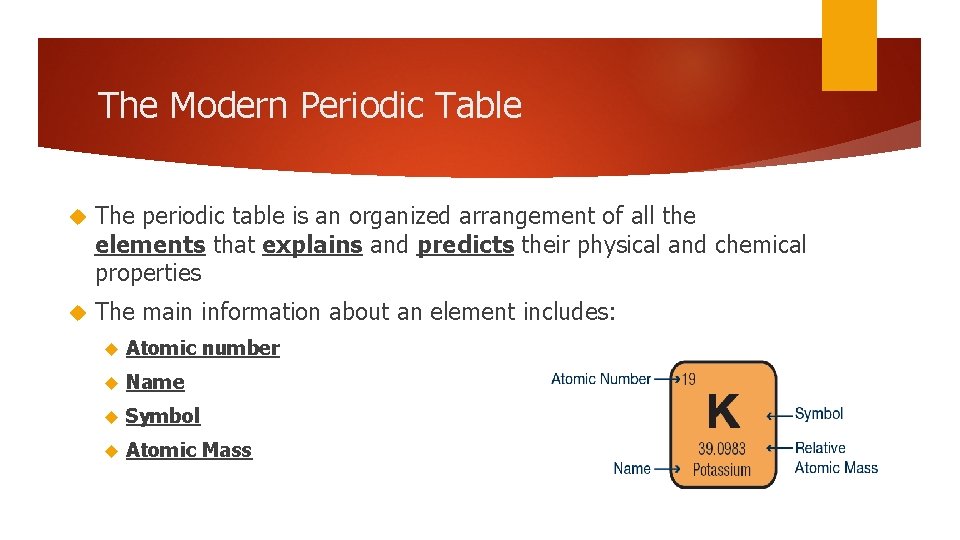
The Modern Periodic Table The periodic table is an organized arrangement of all the elements that explains and predicts their physical and chemical properties The main information about an element includes: Atomic number Name Symbol Atomic Mass
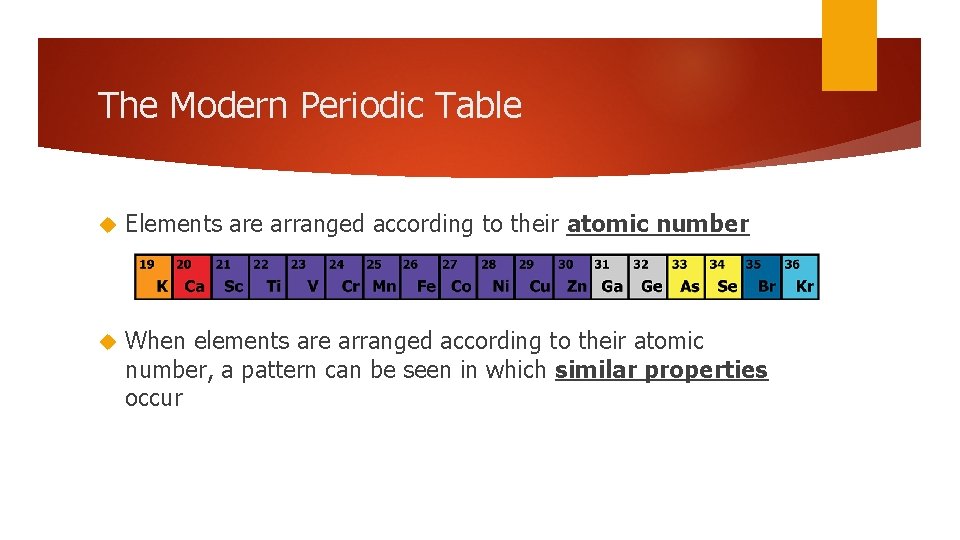
The Modern Periodic Table Elements are arranged according to their atomic number When elements are arranged according to their atomic number, a pattern can be seen in which similar properties occur
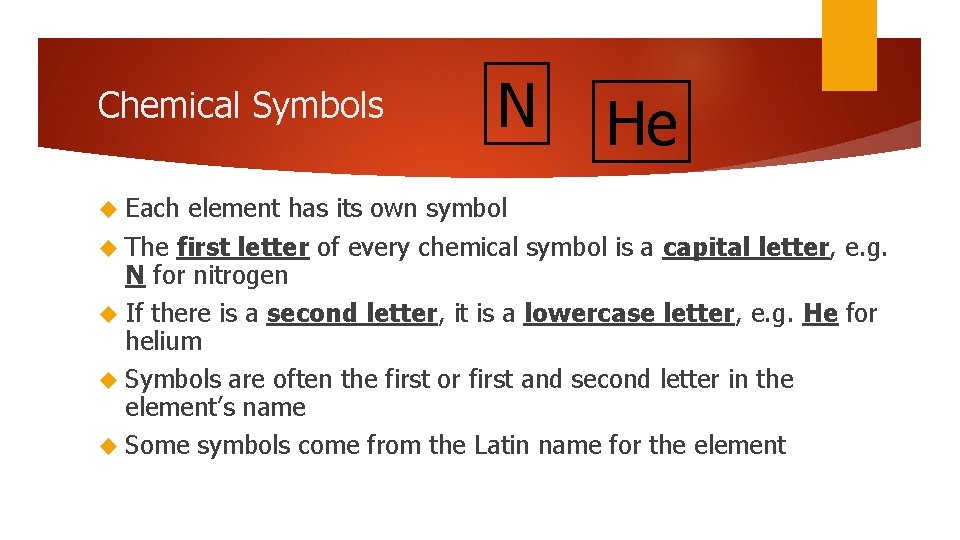
Chemical Symbols Each N He element has its own symbol The first letter of every chemical symbol is a capital letter, e. g. N for nitrogen If there is a second letter, it is a lowercase letter, e. g. He for helium Symbols are often the first or first and second letter in the element’s name Some symbols come from the Latin name for the element
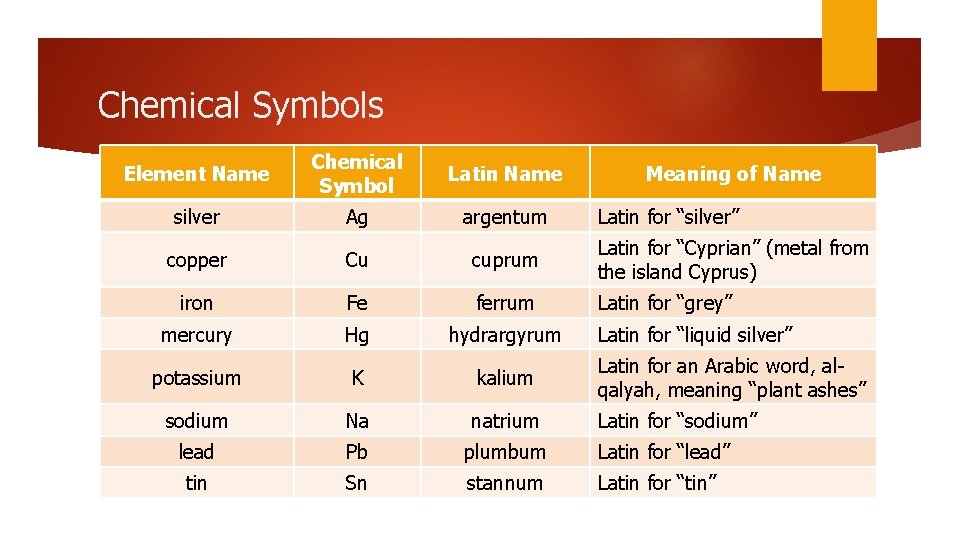
Chemical Symbols Element Name Chemical Symbol Latin Name silver Ag argentum copper Cu cuprum Latin for “Cyprian” (metal from the island Cyprus) iron Fe ferrum Latin for “grey” mercury Hg hydrargyrum potassium K kalium Latin for an Arabic word, alqalyah, meaning “plant ashes” sodium Na natrium Latin for “sodium” lead Pb plumbum Latin for “lead” tin Sn stannum Latin for “tin” Meaning of Name Latin for “silver” Latin for “liquid silver”
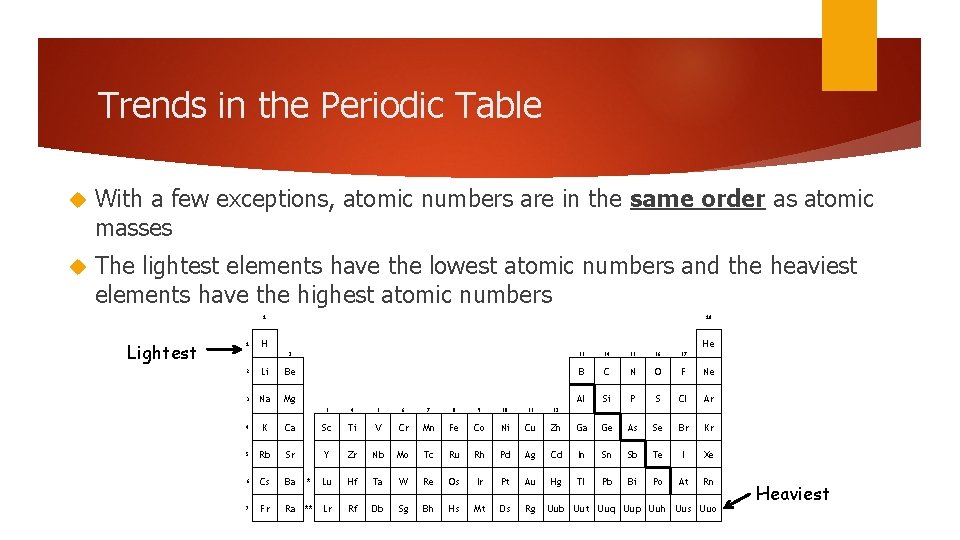
Trends in the Periodic Table With a few exceptions, atomic numbers are in the same order as atomic masses The lightest elements have the lowest atomic numbers and the heaviest elements have the highest atomic numbers 1 Lightest 1 18 H He 2 13 14 15 16 17 B C N O F Ne Al Si P S Cl Ar 2 Li Be 3 Na Mg 3 4 5 6 7 8 9 10 11 12 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 5 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 6 Cs Ba * Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn 7 Fr Ra ** Lr Rf Db Sg Bh Hs Mt Ds Rg Uub Uut Uuq Uup Uuh Uus Uuo Heaviest
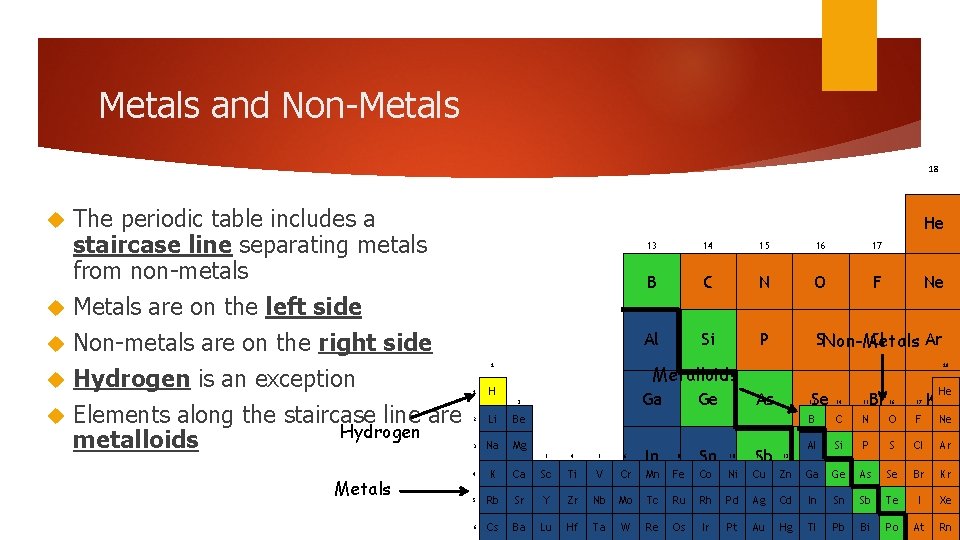
Metals and Non-Metals 18 The periodic table includes a staircase line separating metals from non-metals Metals are on the left side Non-metals are on the right side Hydrogen is an exception Elements along the staircase line are Hydrogen metalloids Metals He 1 1 13 14 15 16 17 B C N O F Al Si P SNon-Metals Cl Ar Ne 18 Metalloids H Ga 2 2 Li Be 3 Na Mg Ge 3 4 5 6 In 7 8 Mn Fe Tc Ru Re Os 4 K Ca Sc Ti V Cr 5 Rb Sr Y Zr Nb Mo 6 Cs Ba Lu Hf Ta W Tl Sn As 9 10 Co Sb Se Br 13 14 15 B C N Al Si P Te I He 16 17 O F S Cl Kr Ne Ar Xe 11 12 Ni Cu Zn Ga Ge As Se Br Kr Rh Pd Ag Cd In Sn Sb Te I Xe Ir Pt Au Hg Tl Pb Bi Po At Rn Rn
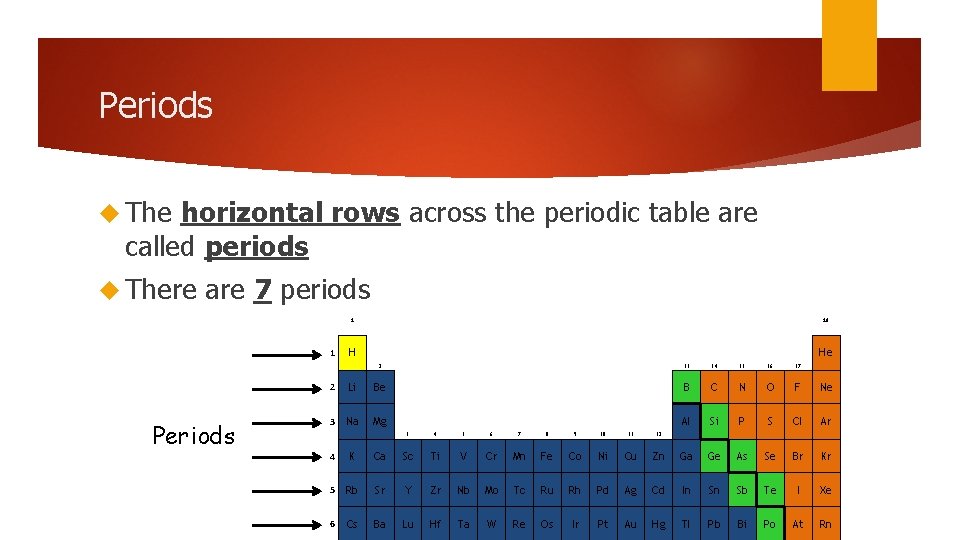
Periods The horizontal rows across the periodic table are called periods There are 7 periods 1 1 Periods 18 H He 2 13 14 15 16 17 B C N O F Ne Al Si P S Cl Ar 2 Li Be 3 Na Mg 3 4 5 6 7 8 9 10 11 12 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 5 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 6 Cs Ba Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
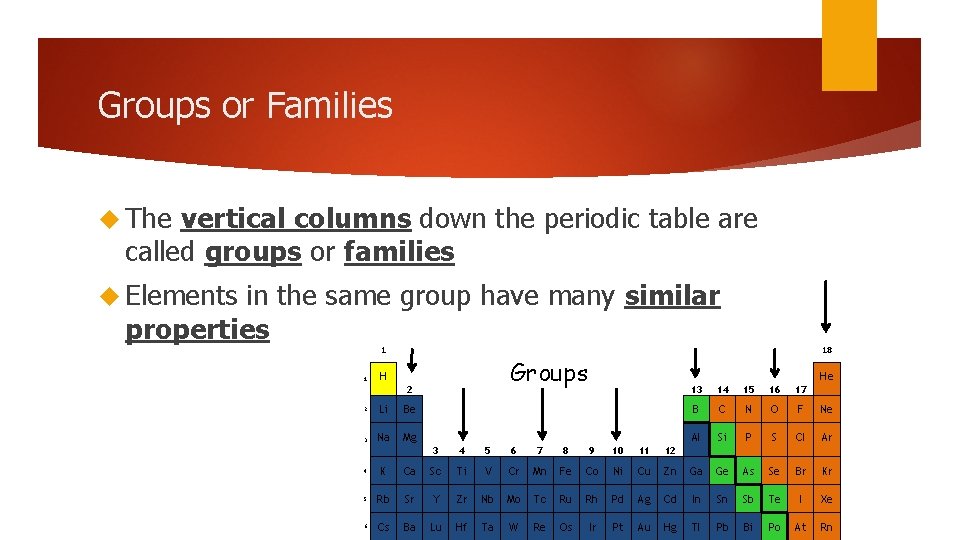
Groups or Families The vertical columns down the periodic table are called groups or families Elements in the same group have many similar properties 1 1 Groups H 2 2 Li Be 3 Na Mg 18 He 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 B C N O F Ne Al Si P S Cl Ar 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 5 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 6 Cs Ba Lu Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
Success Criteria I CAN locate the periods and groups within the periodic table

To Do… Element Names and Symbols Observing Exploring Element the Elements the Periodic Table Poster (after quiz tomorrow)
- Slides: 12