THE PERIODIC TABLE OF THE ELEMENTS Aim How
THE PERIODIC TABLE OF THE ELEMENTS Aim: How can we break down the process of the creation of the periodic table? Do Now: Go to your lab stations

LET’S TRY TO ORGANIZE SOME PICTURES! On each of your lab tables, you will find a large piece of cardboard and a little baggie full of pictures. I would like you to organize those pictures by their color and by their Size
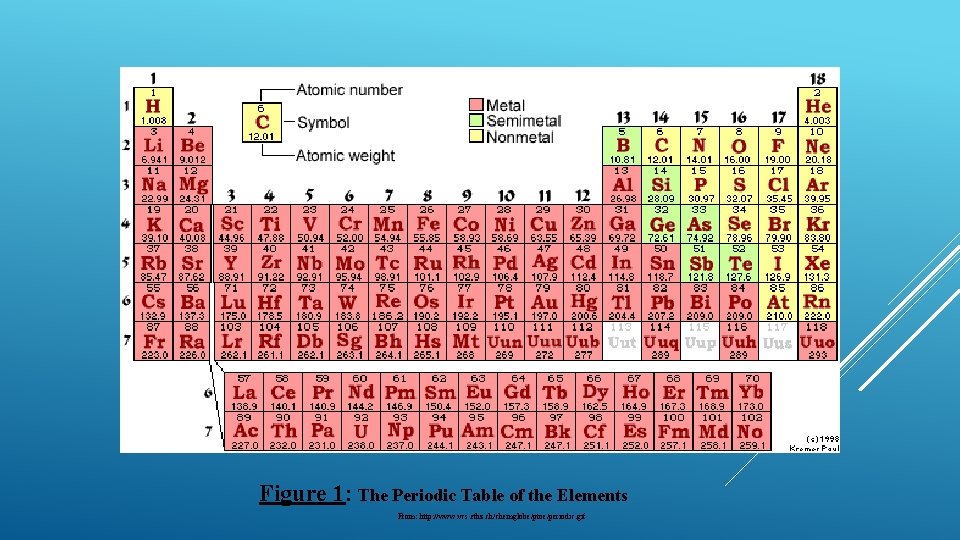
Figure 1: The Periodic Table of the Elements From: http: //www. vcs. ethz. ch/chemglobe/ptoe/periodic. gif
Periodic Table History: 1. Dimitri Mendeleev: He was a Russian chemist who developed an early periodic law. His periodic table was organized based on an element’s atomic mass 1. The issue he had was that there were gaps between different elements AND some of the same elements had different masses (Isotopes!) 2. Henry Moseley: He was a British physicist who changed how the periodic table was organized; he instead organized it by atomic numbers (number of protons) and we still use his model TO THIS DAY!

THERE ARE DIFFERENT MODELS USED TODAY: Example: The alexander arrangement. This table was made to help clear up some inconsistencies with the flat periodic table

MODERN PERIODIC LAW: “the properties of the elements are periodic functions of their atomic numbers”
STRUCTURE OF THE PERIODIC TABLE The periodic table is made up of groups and periods GROUPS: Groups are the vertical part of the periodic table (up and down) Any element in the same group (except groups 3 -12) have the same number of valance electrons Any element in the same group has similar chemical characteristics There are 18 groups on the periodic table PERIODS: Periods are horizontal parts of the periodic table (side to side) Any elements in the same period have the same number of electron shells There are 7 groups on the periodic table
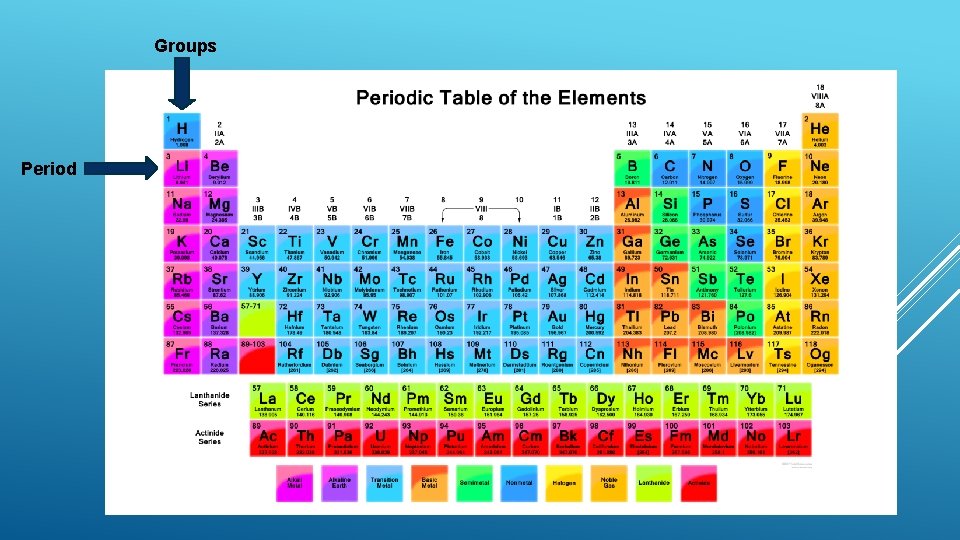
Groups Period

GROUP 1: ALKALI METALS Group 1 metals are the most ACTIVE metals (ex: Potassium and Water) These They metals all have 1 valance electrons have +1 oxidation numbers this They means that they tend to LOSE 1 electron will form cations (positive ions) Francinium table (Fr) is the most active metal on the periodic SIDE NOTE: Hydrogen (H) does not have most of these characteristics; Hydrogen is NOT a metal

GROUP 2: ALKALINE EARTH METALS These metals are active BUT not as active as group one metals They have 2 valance electrons They have +2 oxidation number This means they tend to lose 2 electrons They form positive ions (cations)

GROUPS 3 -11: TRANSITIONAL METALS They are all metals They have multiple oxidation numbers (look at Mn) They form colored aqueous solutions

GROUP 17: HALOGENS These are the most active non-metals Florine is the most active non-metal on the periodic table (F) This is the only group with all three states of matter They have an oxidation of -1 This means that they tend to gain one electron They form anions (negative ions) They have seven valance electrons, They have four diatomic elements (F 2, Cl 2, I 2, Br 2)

GROUP 18: NOBLE GASES They are also known as INERT GASES Inert means they do not react They don’t react because their valance electron shell is FILLED Helium has 2 valance electrons All other Noble gases have 8 valance electrons They are all gases at STP= Standard Temperature and Pressure They can also be referred to as monatomic molecules
WHAT IS THE STATE OF MATTER FOR EACH ELEMENT? The majority of elements are solids and gases There are only TWO elements that are liquids Mercury (Hg)= Liquid Metal Bromine (Br)= Liquid Non-Metal
- Slides: 14