The Periodic Table of Elements Ms Williams 7
The Periodic Table of Elements Ms. Williams 7 th Grade Science Allen Middle School

Dmitri Mendeleev (1834 -1907) • Russian Chemist • Published the first version of the period table in 1869 • Arranged elements according to increasing atomic mass • His periodic table had gaps

Henry Moseley (1887 -1915) • Made improvements to Mendeleev’s Periodic Table • Arranged elements by atomic number instead of mass • Realized that there were undiscovered elements
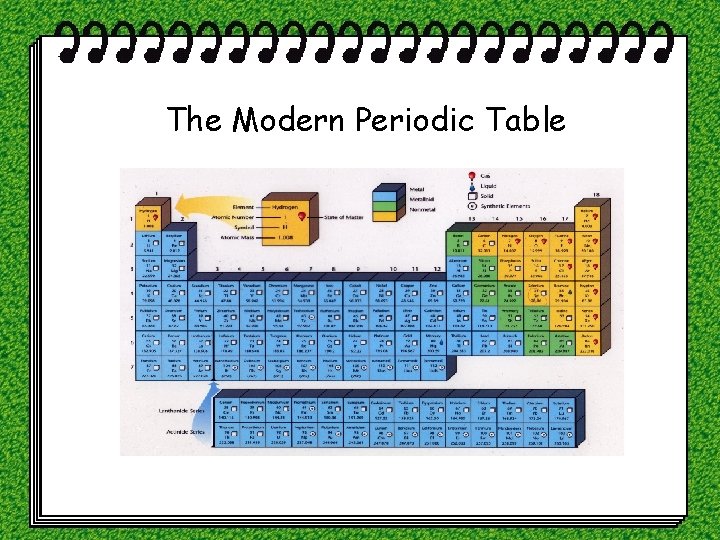
The Modern Periodic Table
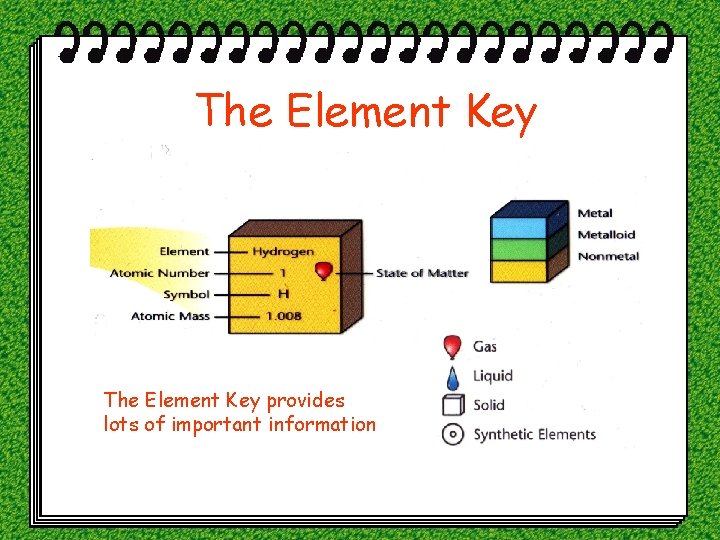
The Element Key provides lots of important information
Time for Vocabulary • Period: A row of elements on a periodic table. Remember rows fly across. • Group: a column of elements on a periodic table that share similar characteristic. Remember groups fall down.
More Vocabulary • Metal: an element that has luster and is a good conductor of heat and electricity. • Nonmetal: elements that are usually gases or brittle solids at room temperature and are poor conductors of heat and electricity. • Metalloid: an element that shares some properties with metals and some with nonmetals.
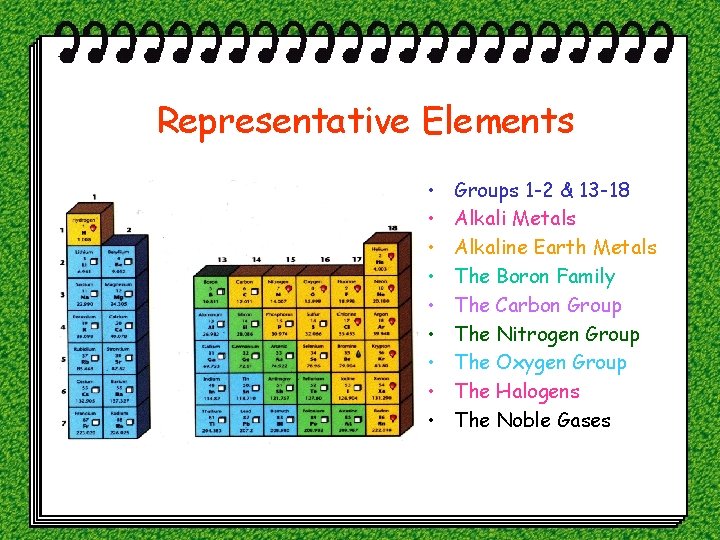
Representative Elements • • • Groups 1 -2 & 13 -18 Alkali Metals Alkaline Earth Metals The Boron Family The Carbon Group The Nitrogen Group The Oxygen Group The Halogens The Noble Gases

The Alkali Metals • Group 1 Elements: -Lithium -Rubidium -Sodium -Cesium -Potassium -Francium • Silvery Solids • Low Densities • Low Melting Points
The Alkaline Earth Metals • The Group 2 Elements -Beryllium -Magnesium -Calcium -Strontium -Barium -Radium • Denser than Alkali Metals • Higher melting points than Alkali Metals
The Boron Family • Group 13 Elements -Boron -Aluminum -Gallium -Indium -Thallium • All are metals except Boron • Aluminum is the most common metal in the Earth’s crust.

The Carbon Group • Group 14 Elements -Carbon -Silicon -Germanium -Tin -Lead • Silicon is used to make semiconductors for computers and other electronics. • Diamond and Graphite are two forms of carbon.

The Nitrogen Group • Group 15 Elements -Nitrogen -Phosphorus -Arsenic -Antimony -Bismuth • Almost 80% of the air we breathe is nitrogen. • Phosphorus is an essential ingredient in healthy teeth and bones.

The Oxygen Group • Group 16 Elements -Oxygen -Sulfur -Selenium -Tellurium -Polonium • About 20% of the Earth’s atmosphere is oxygen. • Sulfuric acid is one the most used chemicals in the world
The Halogens • Group 17 Elements -Fluorine -Chlorine -Bromine -Iodine -Astatine • The Halogens form salts with the alkali metals. • Fluorine is an active ingredient in toothpaste.
The Noble Gases • Group 18 Elements -Helium -Neon -Argon -Krypton -Xenon -Radon • Helium is used to fill balloons. • Neon signs contain noble gases.
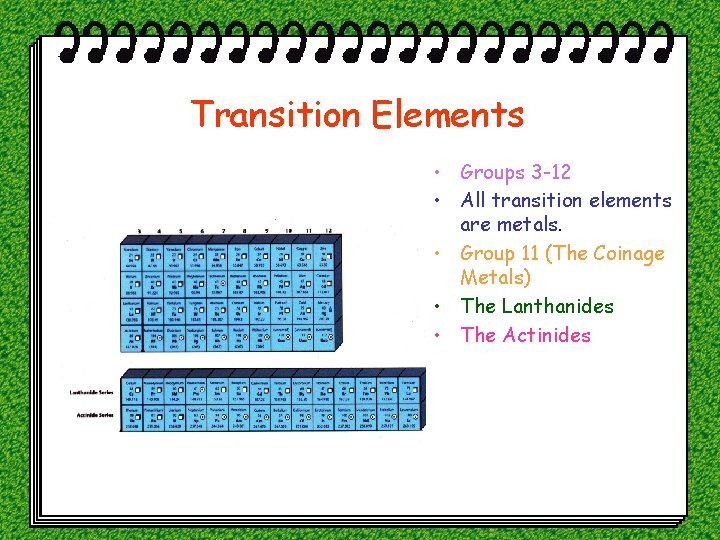
Transition Elements • Groups 3 -12 • All transition elements are metals. • Group 11 (The Coinage Metals) • The Lanthanides • The Actinides

The Coinage Metals • Group 11 Elements -Copper -Gold -Silver • These elements were often used by ancient civilizations to make coins.

The Lanthanides and The Actinides • The Lanthanides • Soft metals that can be cut with a knife. • Were once called rare earth metals. • Glass used in computer and TV screens contain Yttirum and Europium. • The Actinides • All actinides are radioactive. • Thorium, Proactinium and Uranium are the only actinides found naturally on earth. • Plutonium is used to fuel nuclear power plants.
Let’s See What We Remember • What are rows on a periodic table called? • What are columns on a periodic table called? • Name the two divisions of the periodic table? • What can we learn from an element key? • How can scientist use the periodic table?

The End A Comic. Relief Production
- Slides: 21