The Periodic Table of Elements History of the
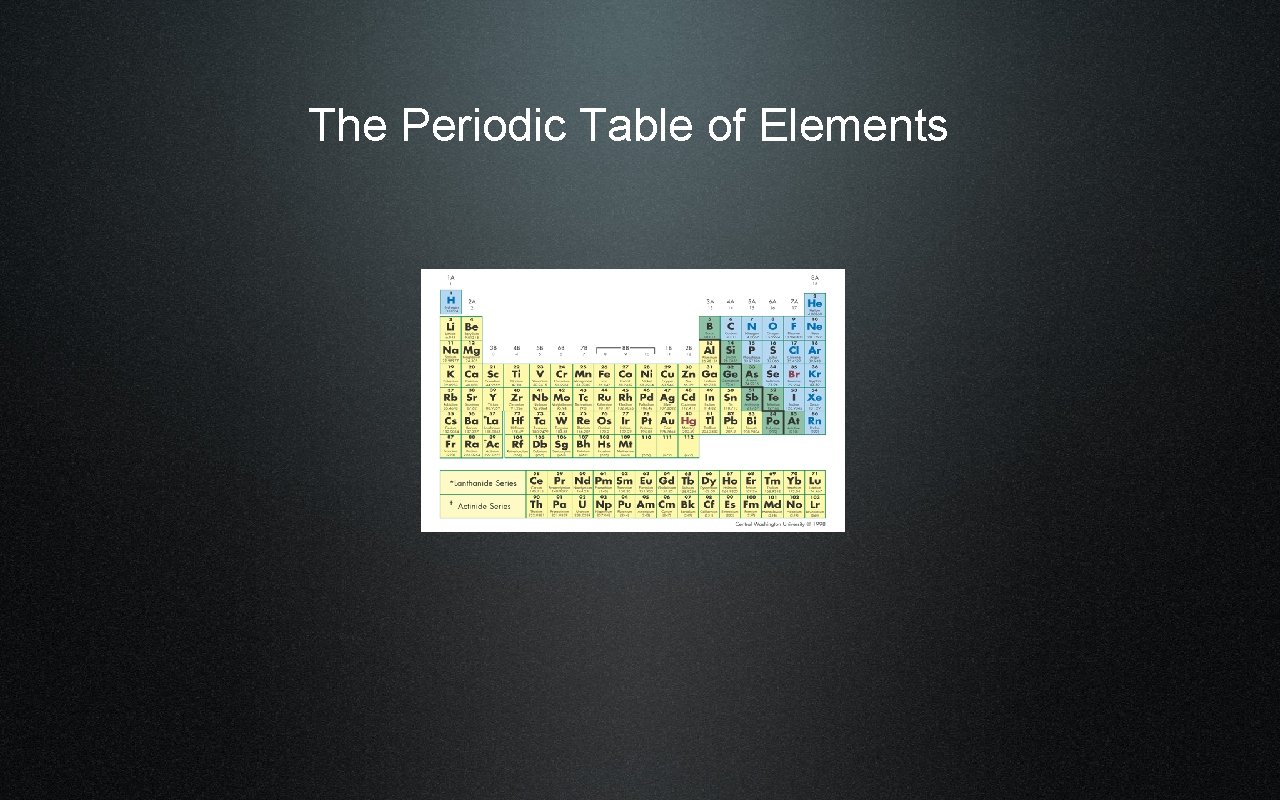
The Periodic Table of Elements

History of the Periodic Table • 1869 : Dmitri Mendeleev • Considered the father of the periodic table of elements • Periodic - happening at regular intervals

Mendeleev’s Table • Organized by atomic mass • Was able to predict the existence of the element Ge based on its properties


Families of the Periodic Table
Alkali Metals • Group 1 or IA • Single e- in outer energy level (valence e-) • Soft, Silvery white & Shiny • The most reactive metals • Never found “free” in nature (always in a cmpd) • Soaps are formed when alkali metals react with fat

What does Mac. Gyver think about alkali metals? ? ?

Alkali metals react violently with water.

Alkaline Earth Metals • Group 2 or IIA • Have 2 valence e • Not as reactive as alkali metals • Produce strong, lightweight alloys • Never found “free” in nature
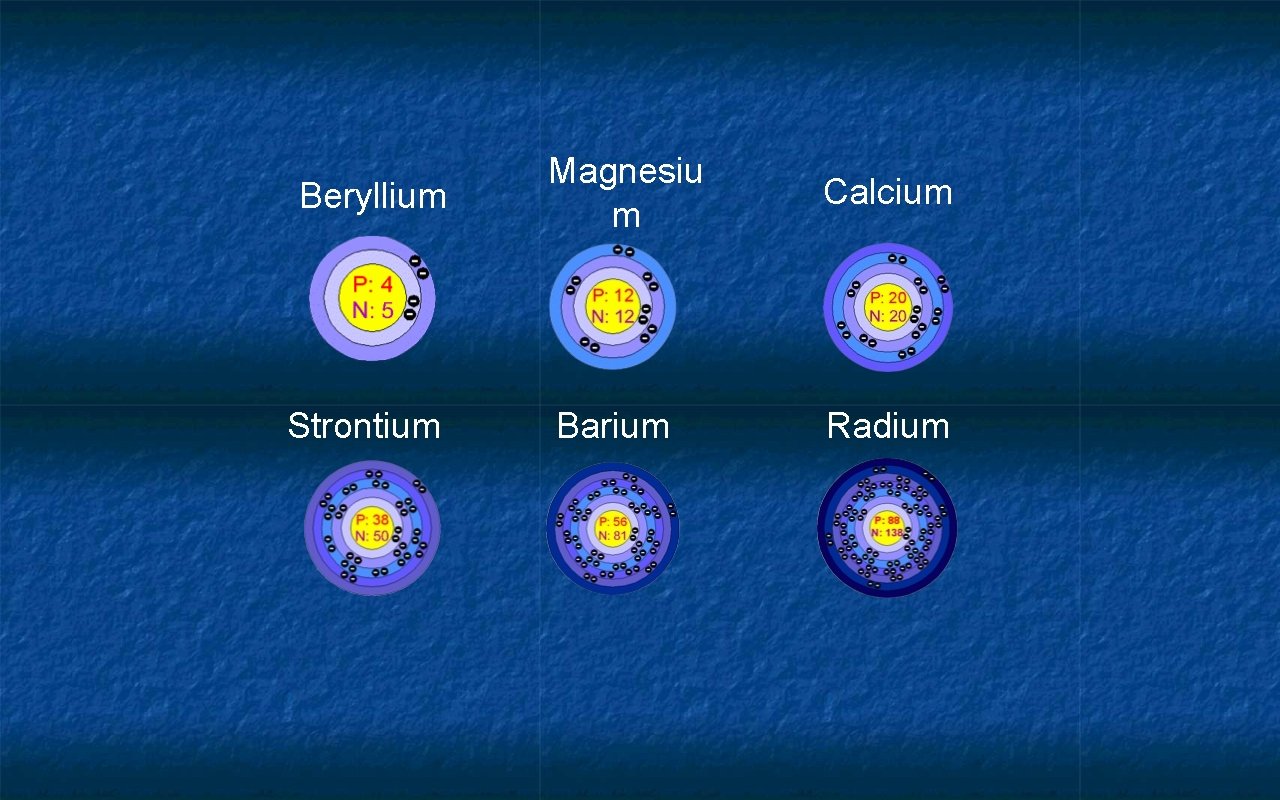
Beryllium Strontium Magnesiu m Calcium Barium Radium
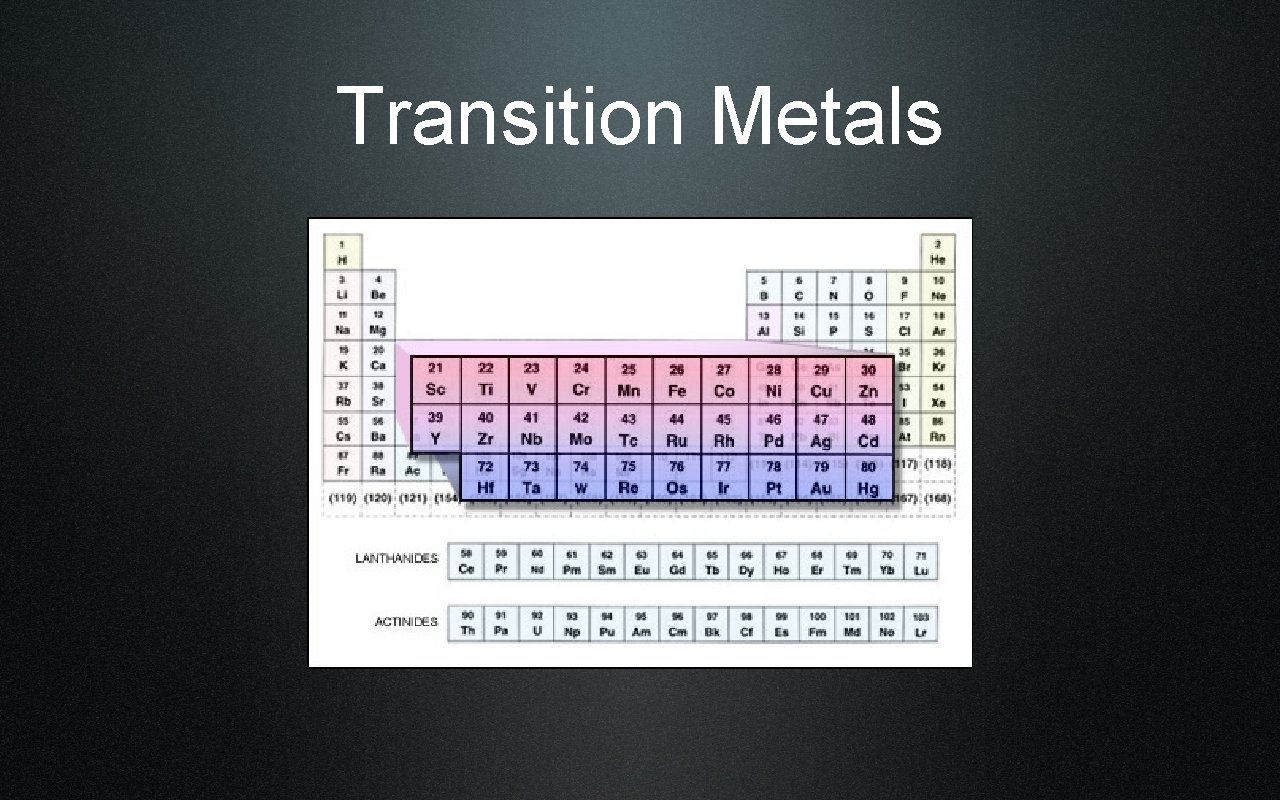
Transition Metals
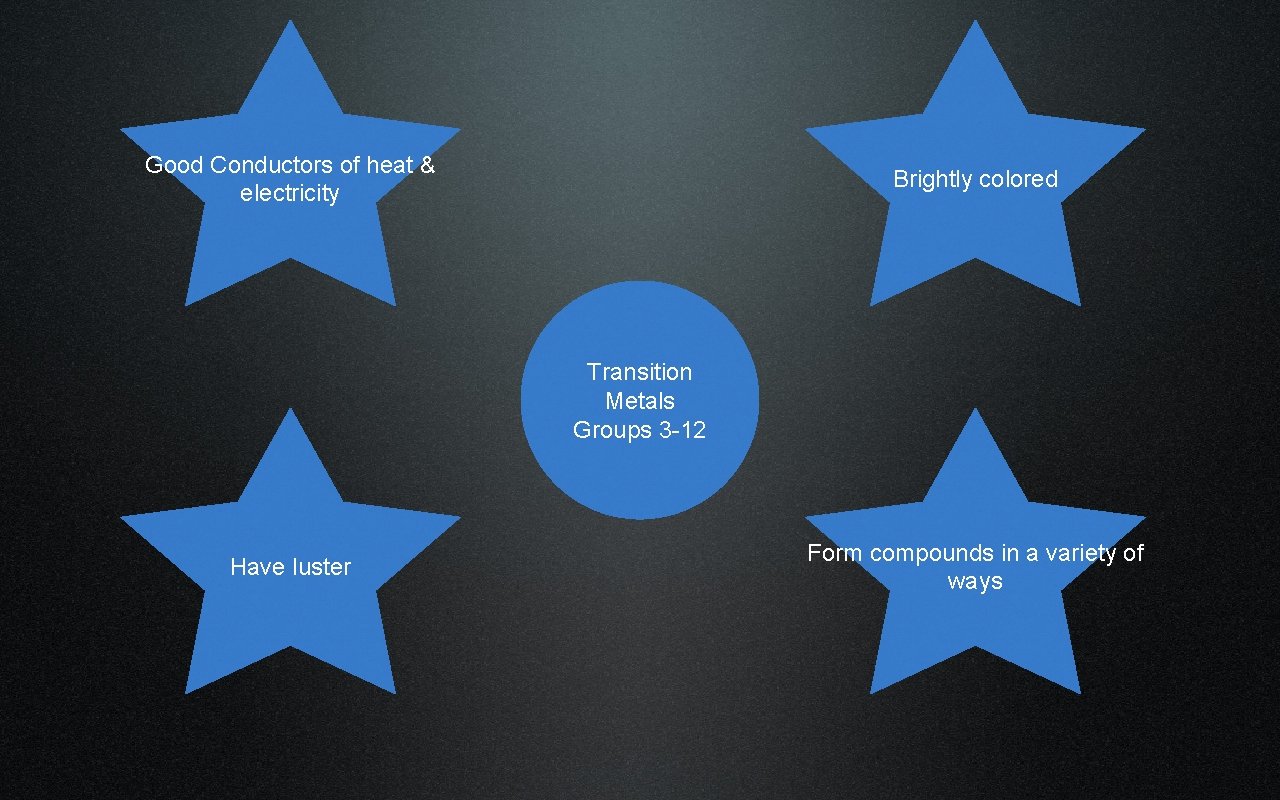
Good Conductors of heat & electricity Brightly colored Transition Metals Groups 3 -12 Have luster Form compounds in a variety of ways

Common Transition Metals Steel Gold & Silver
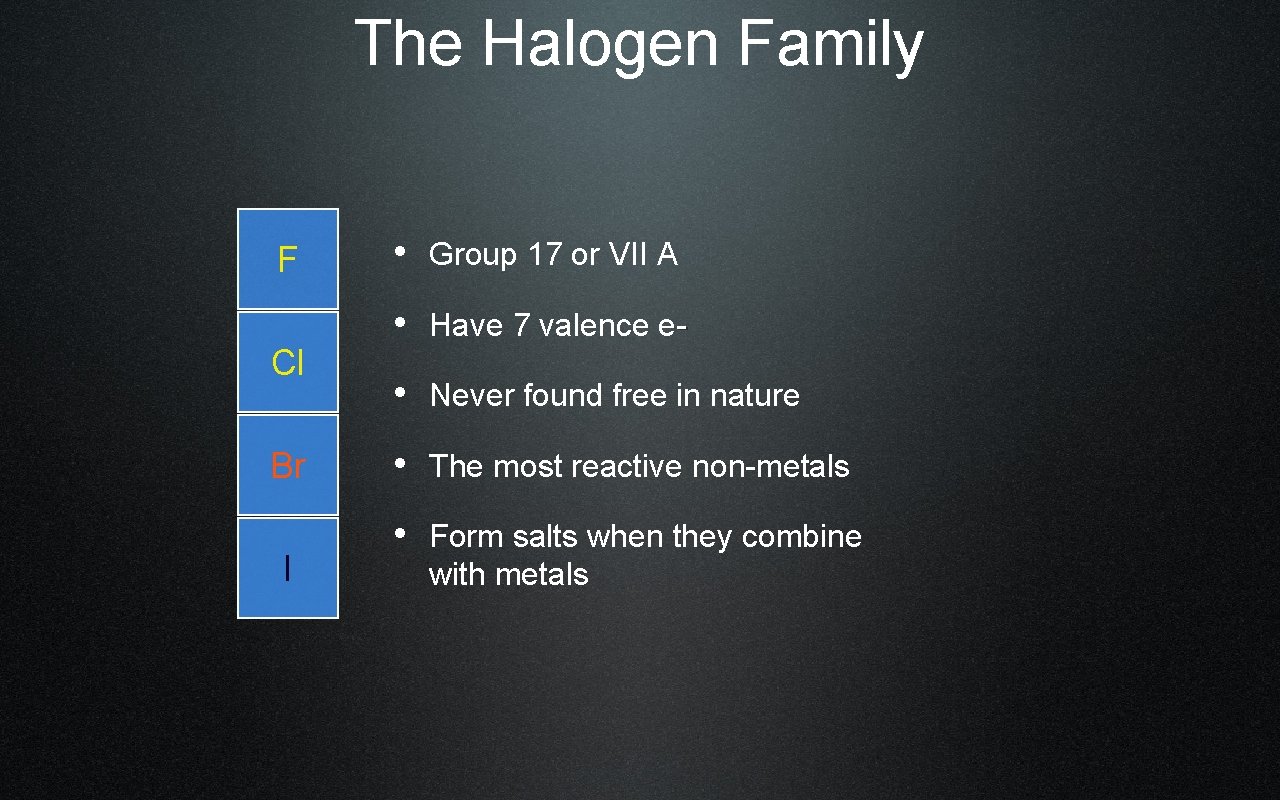
The Halogen Family F Cl Br I • Group 17 or VII A • Have 7 valence e • Never found free in nature • The most reactive non-metals • Form salts when they combine with metals

Properties/Reactivity of the Halogens

The Inert Gas Family • Also called the Noble Gas Family • Group 18 or VIIIA • Have 8 valence e • Considered un-reactive • Glow bright colors when energized • `

Noble Gases are used to make Noble Gases differ in density neon signs
- Slides: 17