The Periodic Table of elements Elements in Earths
The Periodic Table of elements
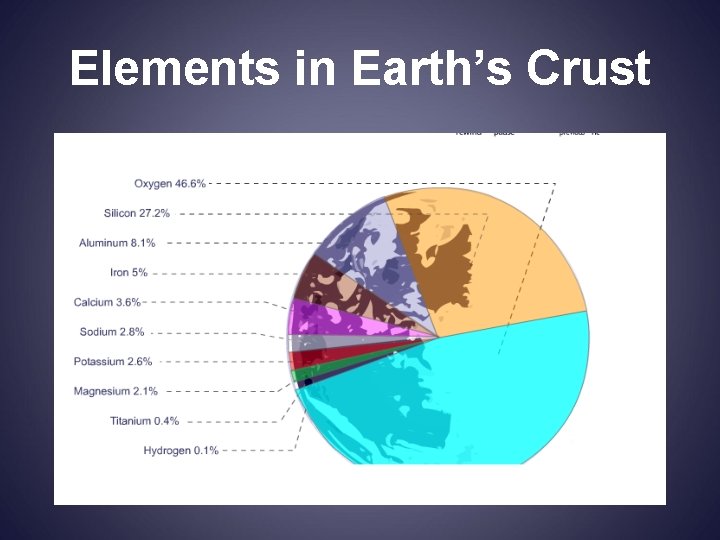
Elements in Earth’s Crust
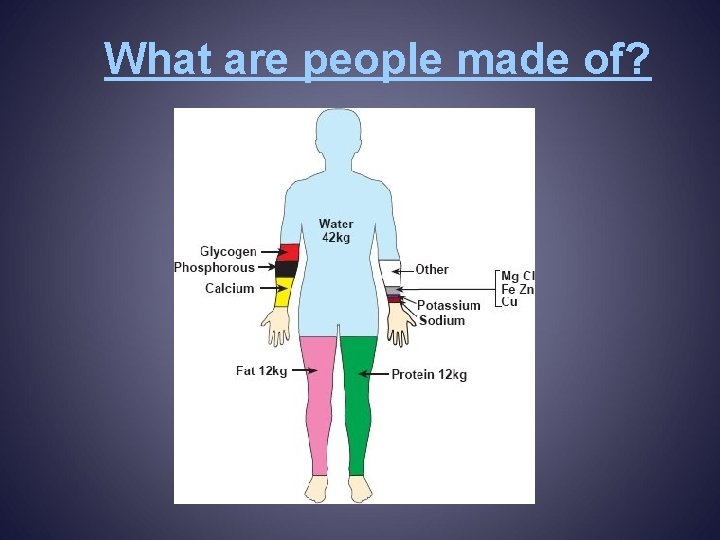
What are people made of?

Mendeleev- First Periodic Table ( 1869) • Grouped by atomic mass & chemical properties. • Blank spaces were left to add predicted elements

Mendeleev’s Periodic Table

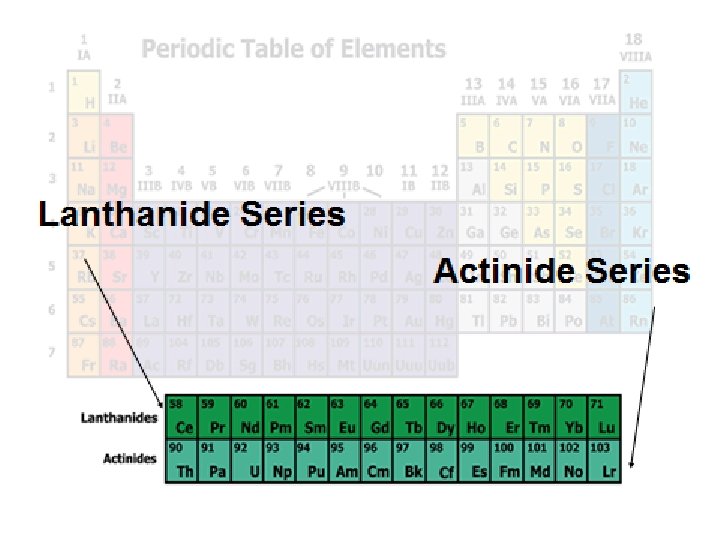
Mosley 1914 - Modern P. T. • Atomic # • Similar property elements ended up next to each other • Periodic Law

Periodic Law Repetition of physical & chemical properties due ↑ atomic #
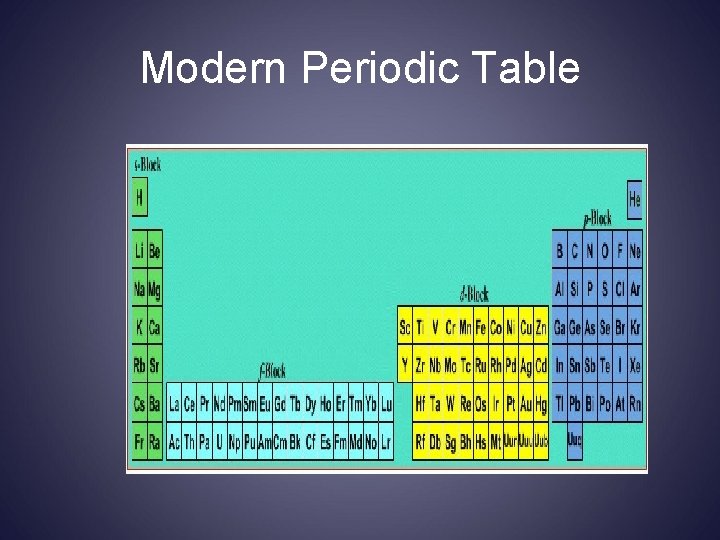
Modern Periodic Table
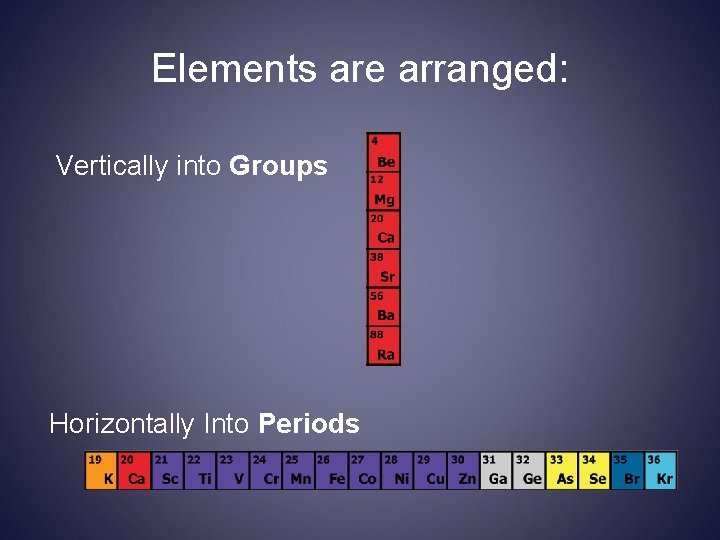
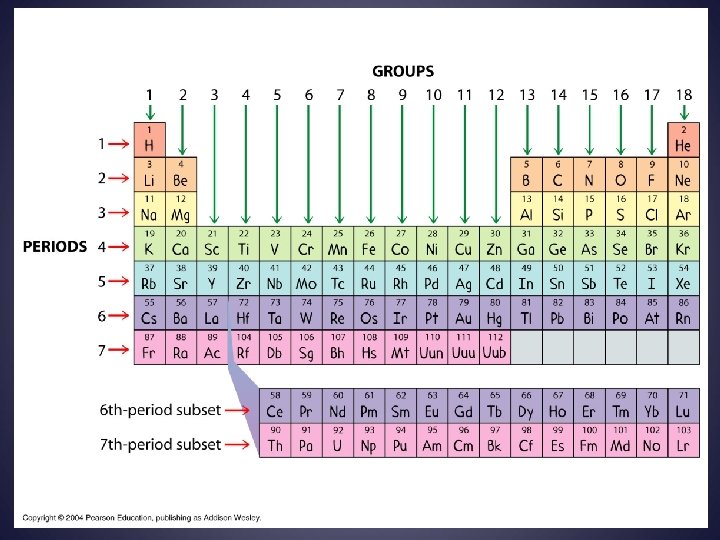
Elements are arranged: Vertically into Groups Horizontally Into Periods

Why?

If you looked at an atom of each element in a group, you would see…
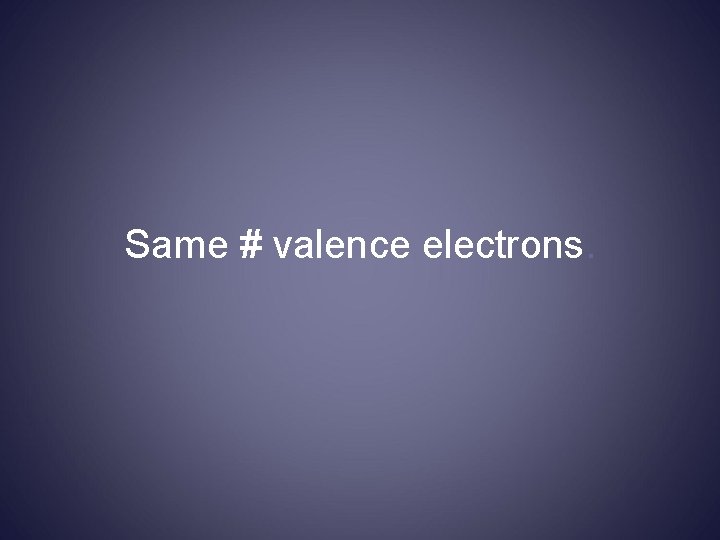
Same # valence electrons.
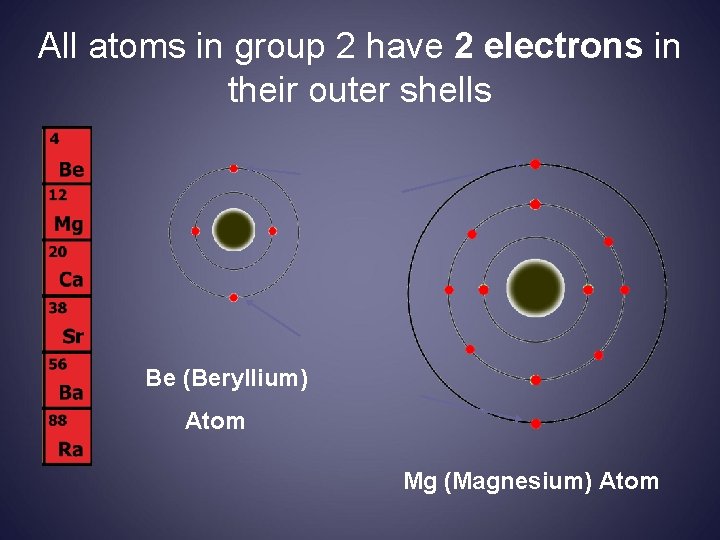
All atoms in group 2 have 2 electrons in their outer shells Be (Beryllium) Atom Mg (Magnesium) Atom
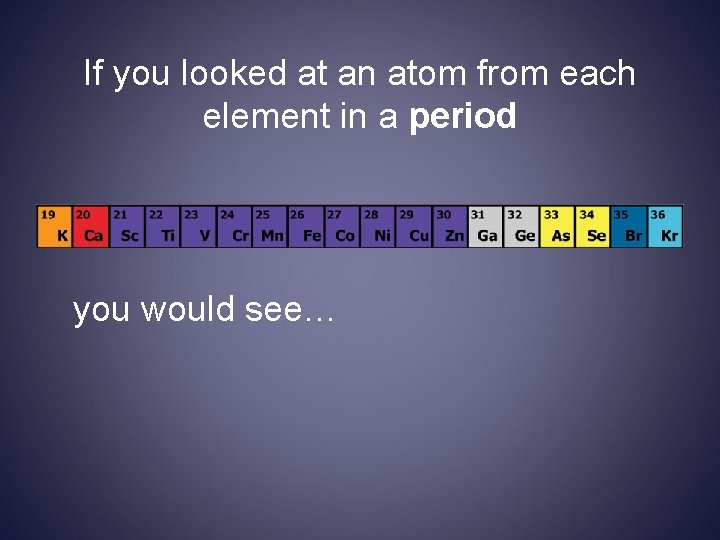
If you looked at an atom from each element in a period you would see…
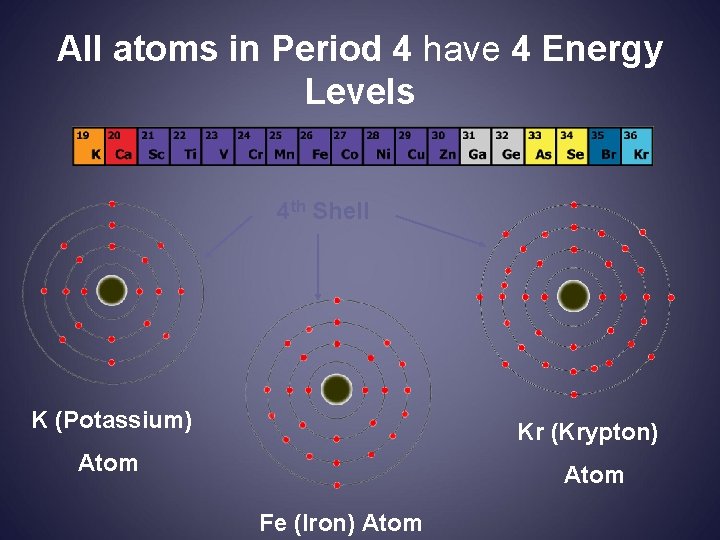
All atoms in Period 4 have 4 Energy Levels 4 th Shell K (Potassium) Kr (Krypton) Atom Fe (Iron) Atom
Shells = Ring= Orbit = E Level
Periodic Patterns Element’s chemical behavior is determined by: its e- config

Each Group has distinct properties

Alkali Metals • 1 valence e-: ns 1 • Most reactive metals • Always bonded with another element.

Alkaline Earth Metals • 2 valence e-: ns 2 • Never found free in nature • Found in rocks/ earth’s crust
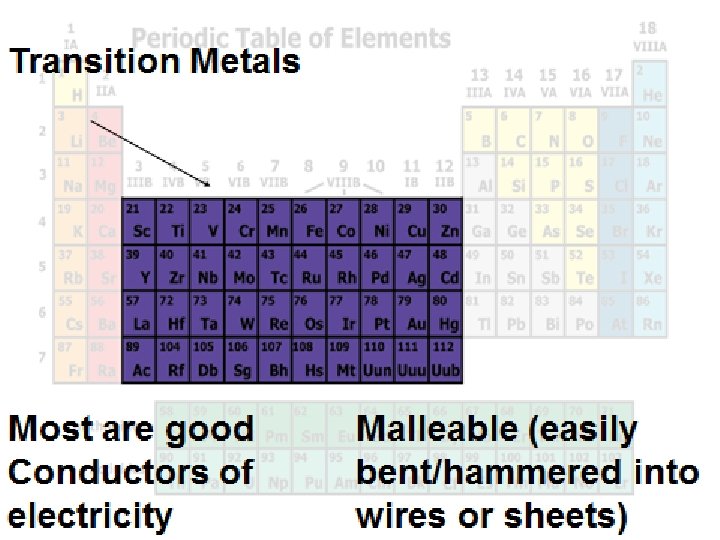

How many things can you think of that have Transition Metals in them?


Hydrogen • In a class of its own • Gas @ room temp.

Group 13: 3 valence e. Group 14: 4 valence e. Group 15: 5 valence e. Group 16: 6 valence e- ns 2 np 1 ns 2 np 2 ns 2 np 3 ns 2 np 4

Interesting facts • Carbon element of life • Nitrogen 78% atmosphere • Oxygen the most abundant element in earth’s crust

Halogen • • 7 valence electrons ns 2 np 5 Most active non-metals. Never found free in nature Poisonous

Noble Gases • • • Very stable 8 valence electrons ns 2 np 6 Colorless gases Un reactive (inert) World’s running out of He
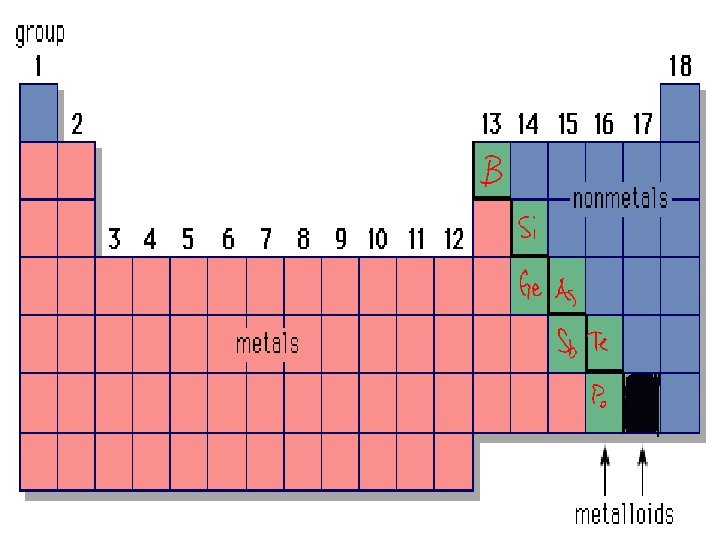

Properties of Metals 1. Conductors of heat and electricity 2. Luster: shiny. 3. Ductile: stretched into thin wires 4. Malleable: pounded into thin sheets

Properties of Metalloids • Metalloids : metal-like • Can be shiny or dull • Conduct heat/electricity better than non-metals but not as well as metals. • They are ductile and malleable.

What are metalloids used for?


Properties of Non-Metals • Not: ductile, malleable, shiny, or conductor • Brittle and break easily

ELEMENT SONG


Element Game
- Slides: 40