THE PERIODIC TABLE OF ELEMENTS ATOMIC THEORISTS This

THE PERIODIC TABLE OF ELEMENTS

ATOMIC THEORISTS

This handsome fellow, John Dalton, Not only researched and made important atomic discoveries, but he also was the first man to hypothesize that colour-blindness was hereditary – and he was right!

This is JJ Thompson. JJ’s favourite activities were playing with electricity, winning the Nobel Prize and he was buried beside Sir Isaac Newton in Westminster Abbey

Ernest Rutherford was a Kiwi, who became known as the “father of nuclear physics”.

Niels Bohr was anything but one… He made contributions to the fields of chemistry, physics and philosophy, had a brother and a son who went to the Olympics, and he fled the Nazis… and somewhere in there he did something to get an element named after himself.

LEARNING GOALS I will be able to show trends in the organization of the elements in the periodic table I will PERSEVERE and keep trying even on difficult tasks

PATTERNS AMONG THE ELEMENTS • By the 1700 s Chemists had gathered a lot of information about elements, however they still had many questions: • Why are some elements gases and others are metals? • How many elements are there? • What relationships can be found between elements? • In 1867, Russian Chemist Dmitri Mendeleev proposed organizing information about the elements into a table. • He gathered all of the information he could about the known elements and wrote it down on cards. One element per card. • He gathered information such as: color, density, melting point and reactivity. • He then sorted the cards into rows and columns based on similarities in the elements properties.
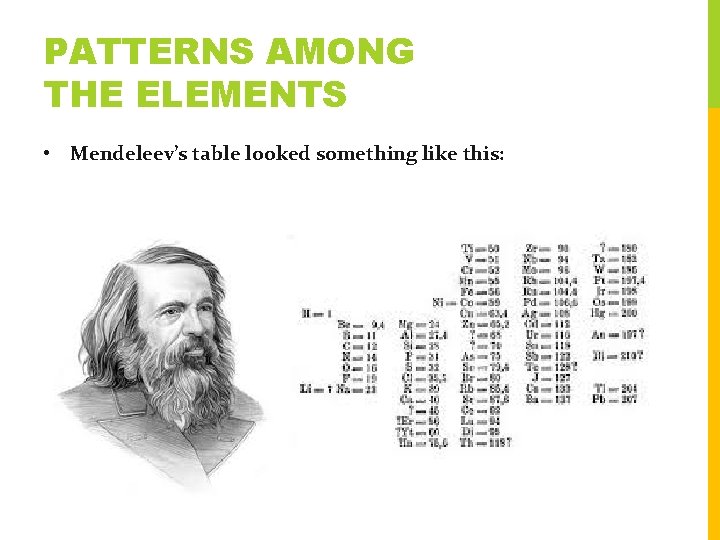
PATTERNS AMONG THE ELEMENTS • Mendeleev’s table looked something like this:

UNKNOWN ELEMENT INQUIRY Each Ziploc bag has a group of ELEMENTS with a description of properties First 10 minutes sort WITHOUT looking at periodic table I will let you know when you can use the information on the back of your handout to order the elements Look for TRENDS in properties What are the UNKNOWN ELEMENTS?
PATTERNS AMONG THE ELEMENTS • Mendeleev’s patterns were so accurate that he was able to leave gaps where he believed undiscovered elements would fit. • As time went on Scientists were able to fill in the gaps, proving Mendeleev’s predictions. • Later, the periodic table was rearranged by Henry Moseley. It is now arranged according to atomic number. The Periodic Table: Crash Course #4 https: //www. youtube. com/watch? v=0 RRVV 4 Diomg
THE MODERN PERIODIC TABLE • The periodic table of elements is a chart that places all of the elements in rows and columns. • The elements are organized according to their atomic number. • The rows are called periods and the columns are called groups or families. • The elements found in each group or family share some common characteristics. Groups/Families

ELEMENTS Elements can be divided into two categories : Metals and Non. Metals tend to be: • • • Hard (at room temperature) Shiny Malleable (can be pounded into sheets) Ductile (can be stretched into wires) Good conductors of heat and electricity Non-metals tend to be: • Gases or brittle (crumbly or breakable) solids at room temperature
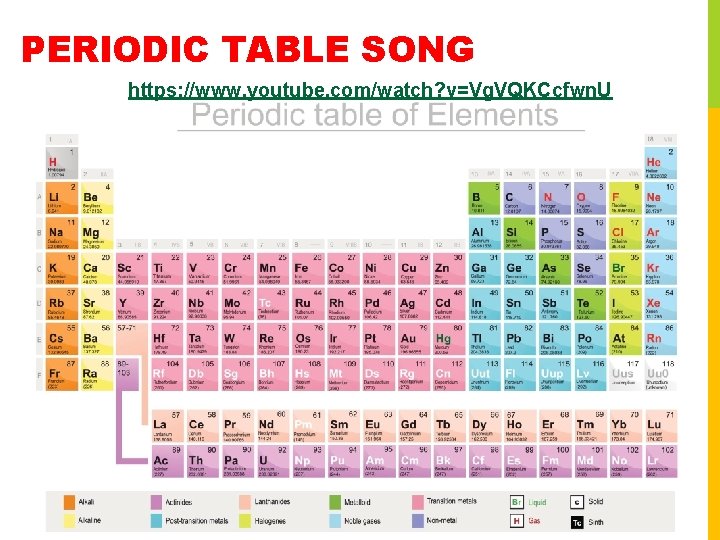
PERIODIC TABLE SONG https: //www. youtube. com/watch? v=Vg. VQKCcfwn. U

PROPERTIES OF METALS Metals are good conductors of heat and electricity. Metals are shiny. Metals are ductile (can be stretched into thin wires). Metals are malleable (can be pounded into thin sheets). A chemical property of metal is its reaction with water which results in corrosion.

PROPERTIES OF NONMETALS Non-metals are poor conductors of heat and electricity. Non-metals are not ductile or malleable. Solid non-metals are brittle and break easily. They are dull. Many non-metals are gases. Sulfur

PROPERTIES OF METALLOIDS Metalloids (metal-like) have properties of both metals and non-metals. They are solids that can be shiny or dull. They conduct heat and electricity better than nonmetals but not as well as metals. Silicon They are ductile and malleable.

FAMILIES • Columns of elements are called groups or families. PERIODS • Each horizontal row of elements is called a period. • Elements in each family have • The elements in a period are not alike in properties. similar but not identical properties. • In fact, the properties change greatly across even given row. • For example, lithium (Li), sodium (Na), potassium (K), • The first element in a period and other members of family is always an extremely active solid. The last element in a IA are all soft, white, shiny period, is always an inactive metals. gas. • All elements in a family have the same number of valence electrons.

HYDROGEN The hydrogen square sits atop Family 1, but it is not a member of that family. Hydrogen is in a class of its own. It’s a gas at room temperature.
ALKALI METALS Alkali Metals (Group 1): Li, Na, K, Rb, Cs The alkali family is found in the first column of the periodic table. They are shiny, have the consistency of clay, and are easily cut with a knife.

ALKALI METALS They are the most reactive metals. They react violently with water. Alkali metals are never found as free elements in nature. They are always bonded with another element.

ALKALINE EARTH METALS Group 2 They are never found uncombined in nature. They are highly reactive, but less so than group 1. Alkaline earth metals include beryllium, magnesium, calcium, strontium, barium, and radium.

TRANSITION METALS Transition Elements include those elements in the middle families. These are the metals you are probably most familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity.

PROPERTIES WITHIN GROUPS Halogens (Group 17): F, Cl, Br, I, At Similarities • Non-metals • Gases • Very reactive Differences • Different colors

HALOGEN FAMILY The elements in this family are fluorine, chlorine, bromine, iodine, and astatine. Halogens are the most active non-metals. They are never found free in nature. They react with alkali metals to form salts.
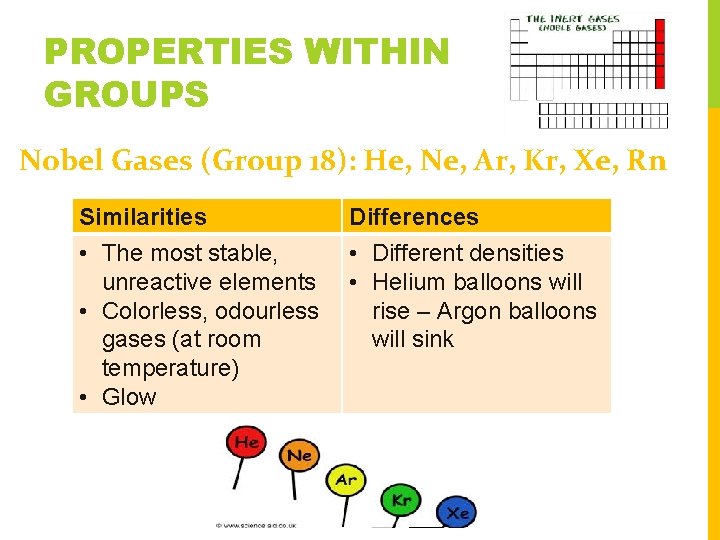
PROPERTIES WITHIN GROUPS Nobel Gases (Group 18): He, Ne, Ar, Kr, Xe, Rn Similarities Differences • The most stable, unreactive elements • Colorless, odourless gases (at room temperature) • Glow • Different densities • Helium balloons will rise – Argon balloons will sink

NOBLE GASES Noble Gases are colorless gases that are extremely un-reactive. One important property of the noble gases is their inactivity. Because they do not readily combine with other elements to form compounds, the noble gases are called inert. The family of noble gases includes helium, neon, argon, krypton, xenon, and radon. All the noble gases are found in small amounts in the earth's atmosphere.
RARE EARTH ELEMENTS The thirty rare earth elements are composed of the lanthanide and actinide series. One element of the lanthanide series and most of the elements in the actinide series are called trans-uranium, which means synthetic or man -made.

ASSIGNMENT Learning Chemical Symbols
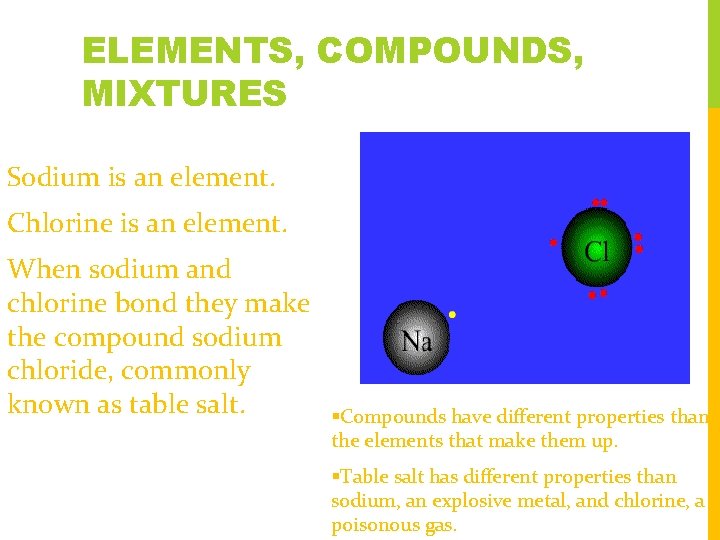
ELEMENTS, COMPOUNDS, MIXTURES Sodium is an element. Chlorine is an element. When sodium and chlorine bond they make the compound sodium chloride, commonly known as table salt. Compounds have different properties than the elements that make them up. Table salt has different properties than sodium, an explosive metal, and chlorine, a poisonous gas.

ELEMENTS, COMPOUNDS, MIXTURES Hydrogen is an element. Oxygen is an element. When hydrogen and oxygen bond they make the compound water. When salt and water are combined, a mixture is created. Compounds in mixtures retain their individual properties. The ocean is a mixture.

ELEMENTS, COMPOUNDS, AND MIXTURES Mixtures can be separated by physical means. Compounds can only be separated by chemical means. Elements are pure substances. When the subatomic particles of an element are separated from its atom, it no longer retains the properties of that element.
- Slides: 32