The Periodic Table n Dmitri Mendeleev credited for

The Periodic Table n Dmitri Mendeleev – credited for the first periodic table in 1869. ¨ He had put element names and a few of their properties on cards and then arranged them in various ways to help his students learn them more easily. ¨ Arranged them so elements in the same column have similar properties.

Periodic Table Song
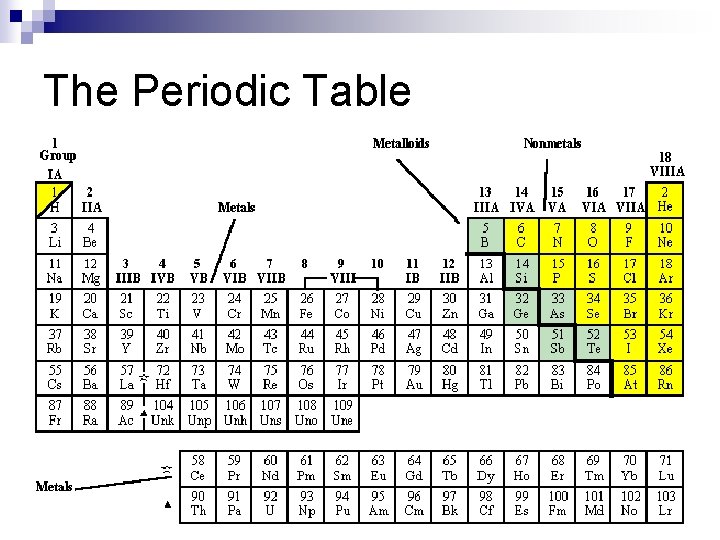
The Periodic Table

n n n n Alkali: Group 1* very reactive Alkaline: Group 2 Boron Family: Group 13 Carbon Family: Group 14 Nitrogen Family: Group 15 Oxygen Family: Group 16 Halogens: Group 17 Noble Gases: Group 18

Regions of the periodic table What do we already know about some of the elements? n List some chemical and physical properties for the following n ¨ Magnesium ¨ Iron ¨ Copper ¨ Carbon ¨ Nitrogen ¨ Iodine ¨ Helium ¨ Silicon

Reading the periodic table n Groups or families – vertical columns n Periods – horizontal rows

The largest region of the periodic table is made of metals. Metals all have similar properties such as: n Good conductors of electricity and heat n Useful in technology n Lustrous n Ductile n Malleable. n Examples: Silver, Mercury, Titanium
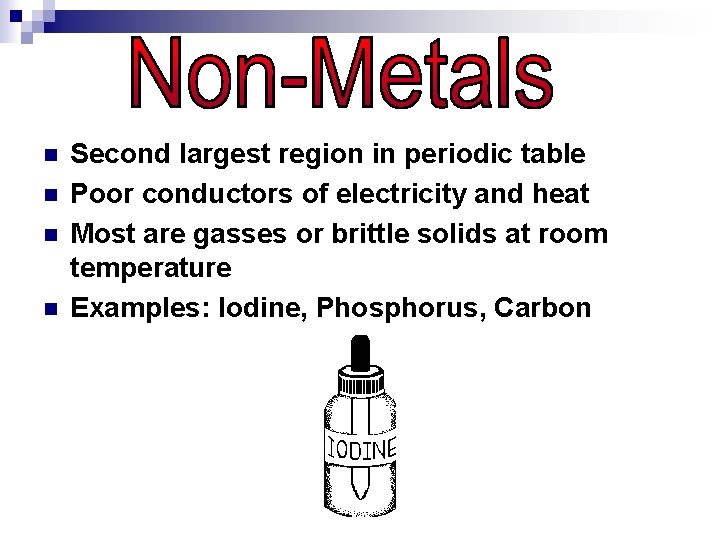
n n Second largest region in periodic table Poor conductors of electricity and heat Most are gasses or brittle solids at room temperature Examples: Iodine, Phosphorus, Carbon

n n n Have properties of metals AND nonmetals Used in semi-conducting devices because it has moderate electrical conductivity Examples: Silicon, Germanium, Arsenic Silicon

n n Very unreactive or inert Rarely form compounds with other elements Can sometimes be used for lighting Examples: Krypton, Neon, Helium Krypton

Trends in reactivity What trends would be predicted for the reaction of the alkali metals with air or water? n Are the reactions going to be similar or different? n

Lab- Mendeleev for a Day n n n Read Lab and Complete the Pre lab Questions Review Procedures Just a reminder ¨ You must wear goggles at all times or you will be asked to leave the lab ~ aprons are optional ¨ Put substances in the appropriate waste containers that are in the hood n Test Solution A- Has Lead in it!!!! ¨ Keep an eye on each other to make sure you are obeying all safety rules.

Discuss Lab n Mendeleev for a Day Based on your observations, group the unknown solutions into families according to similar chemical behavior. Justify your answer with data from the investigation.
Lab Discussion Unknown A- ppt? b-ppt? C-color D- dissolved 1 2 3 4 5 6 7 8 9
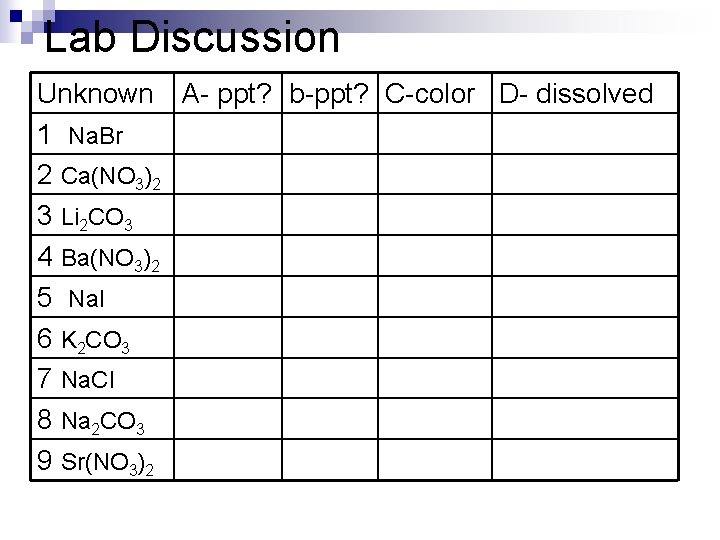
Lab Discussion Unknown A- ppt? b-ppt? C-color D- dissolved 1 Na. Br 2 Ca(NO 3)2 3 Li 2 CO 3 4 Ba(NO 3)2 5 Na. I 6 K 2 CO 3 7 Na. Cl 8 Na 2 CO 3 9 Sr(NO 3)2

Homework n Atomic Structure continued Both sides parts a-d

Periodic Trends
Periodic Trends n Atomic radius n n The distance from the center of an atoms nucleus to it’s outermost electron Measure of atomic size
Periodic Trends Graph the first 20 elements. What is the trend down a group? Across a Period? n Atomic radius

Periodic Trends n Ionic Size n n n Size of an atom when electrons are added or removed. Electrons removed atom becomes smaller. Electrons added atoms become larger
Periodic Trends Graph the first 20 elements. What is the trend down a group? Across a Period? n Ionic Size

Periodic Trends n Ionization Energy needed to remove one of the electrons on an atom’s outer shell. How strongly does an atom hold it’s outermost electron.
Periodic Trends Graph the first 20 elements. What is the trend down a group? Across a Period? n Ionization Energy
Periodic Trends n Electronegativity n n Ability of an atom to attract electrons Which elements want electrons the most?
Periodic Trends Graph the first 20 elements. What is the trend down a group? Across a Period? n Electronegativity

Metal/Non. Metal Trends

Why? Group and Period Trends
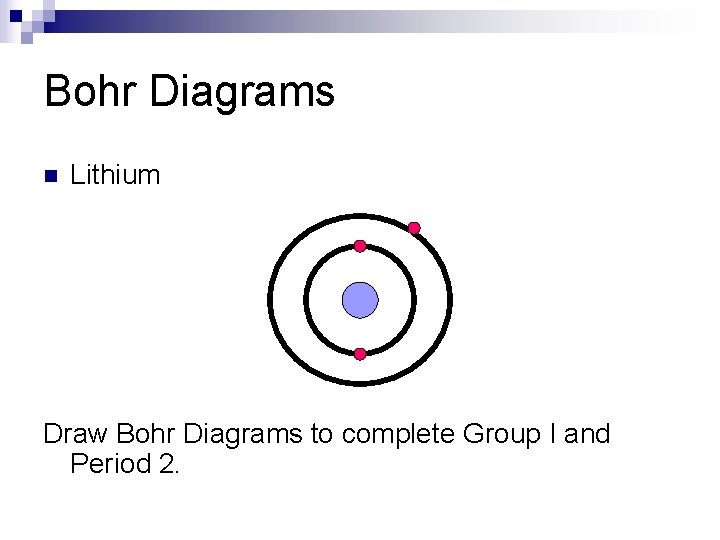
Bohr Diagrams n Lithium Draw Bohr Diagrams to complete Group I and Period 2.

Atomic Radius n Group Trend ¨ Increases from top to bottom ¨ More energy levels or quantum levels as you go down a group – atomic radius increases n Period Trend ¨ Increases from right to left ¨ All electrons in the same energy level. Increased # of protons holds them closer to nucleus.

Ionic Size n Group Trend ¨ Increases from top to bottom ¨ More energy levels as you go down a group – ionic size increases n Period Trend ¨ Decreases as atoms lose more electrons, increases dramatically as atoms start gaining electrons, decreases as atoms gain fewer electrons.

Ionization Energy n Group Trends ¨ Increases from bottom to top. ¨ The closer outer shell electrons are to the nucleus the harder they are to remove. n Period Trend ¨ Increases from left to right. ¨ The more electrons in the outer shell the harder it is to remove one.

Electronegativity n Group Trend ¨ Increases from bottom to top ¨ The closer the outer shell electrons are to the nucleus the more they want electrons n Period Trend ¨ Increases from left to right ¨ The more electrons in the outer shell (up to 7) the more the atom wants electrons

Practice 1. Se and Br 1. 2. P, S, Se 1. 2. 3. Largest atom Highest Ionization Energy Cl, Cl 1 -, Br 11. 4. Smallest atom Lowest Ionization Energy Largest ionic size Mg, Mg 2+, Na 1+ 1. Smallest ionic size

Rally Table

Electron Configurations and Periodic Trends Write the electron configuration and draw an orbital diagram for each element n Order each group of elements or ions based on given data for each property requested on card n Use the orbital diagrams to explain the pattern. (does it agree with the “trend”) n

Objectives Use the periodic table to write electron configurations n Use the periodic table to obtain information about the properties of elements n
- Slides: 36