THE PERIODIC TABLE Mr Coffey The Periodic Table

THE PERIODIC TABLE! Mr. Coffey
The Periodic Table of Elements • Used to keep track of the different elements that are natural & man made • Mendeleev used atomic mass. • The modern Periodic Table uses atomic number
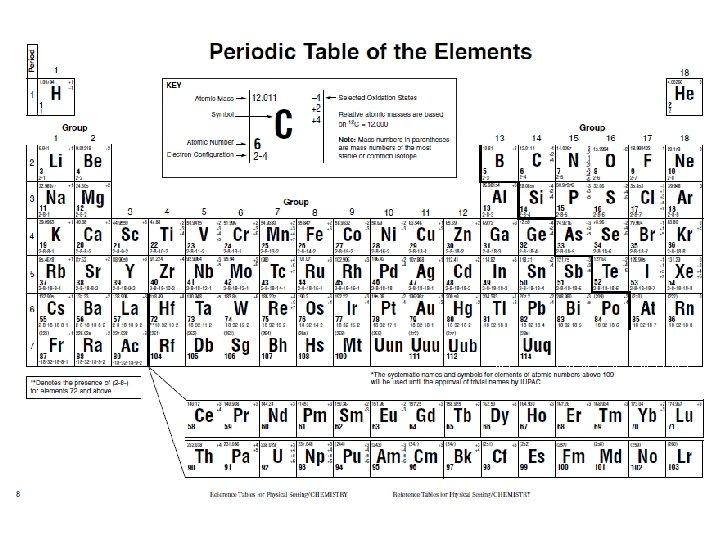
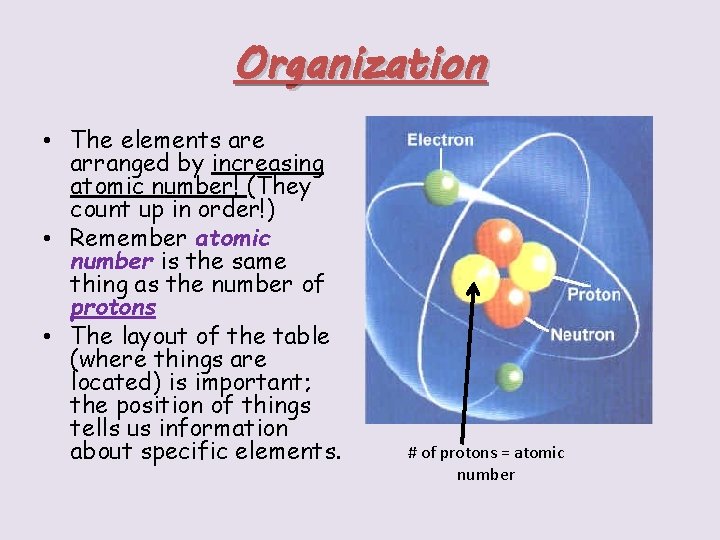
Organization • The elements are arranged by increasing atomic number! (They count up in order!) • Remember atomic number is the same thing as the number of protons • The layout of the table (where things are located) is important; the position of things tells us information about specific elements. # of protons = atomic number
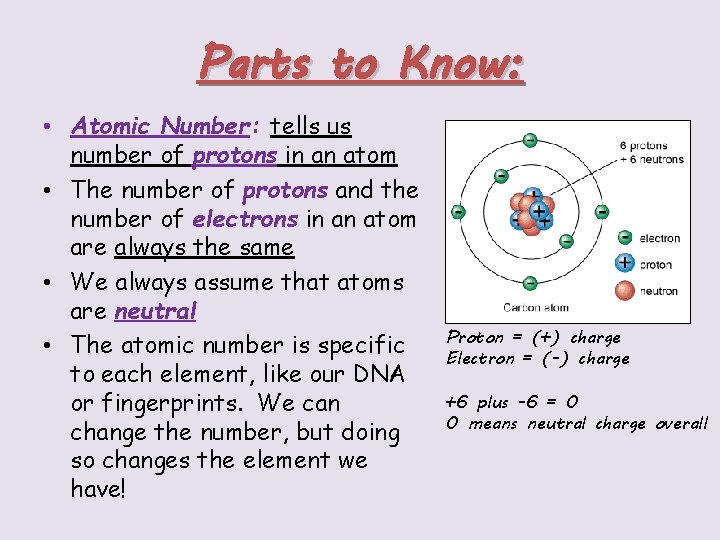
Parts to Know: • Atomic Number: tells us number of protons in an atom • The number of protons and the number of electrons in an atom are always the same • We always assume that atoms are neutral • The atomic number is specific to each element, like our DNA or fingerprints. We can change the number, but doing so changes the element we have! Proton = (+) charge Electron = (-) charge +6 plus -6 = 0 0 means neutral charge overall
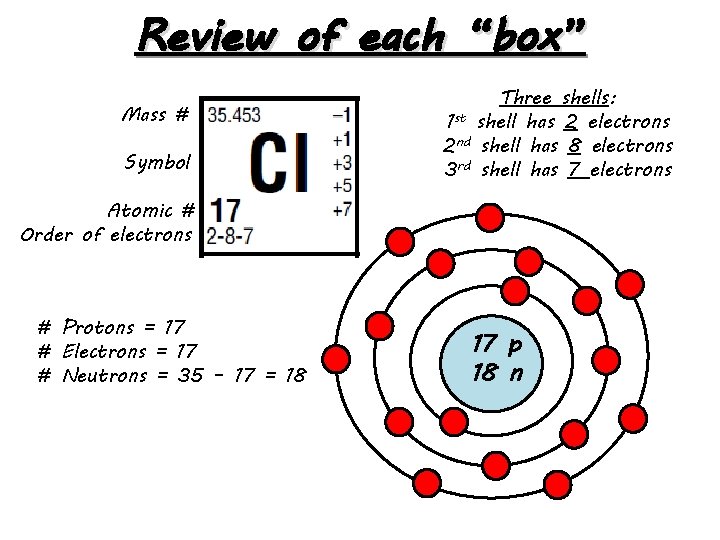
Review of each “box” Mass # Symbol Three shells: 1 st shell has 2 electrons 2 nd shell has 8 electrons 3 rd shell has 7 electrons Atomic # Order of electrons # Protons = 17 # Electrons = 17 # Neutrons = 35 – 17 = 18 17 p 18 n
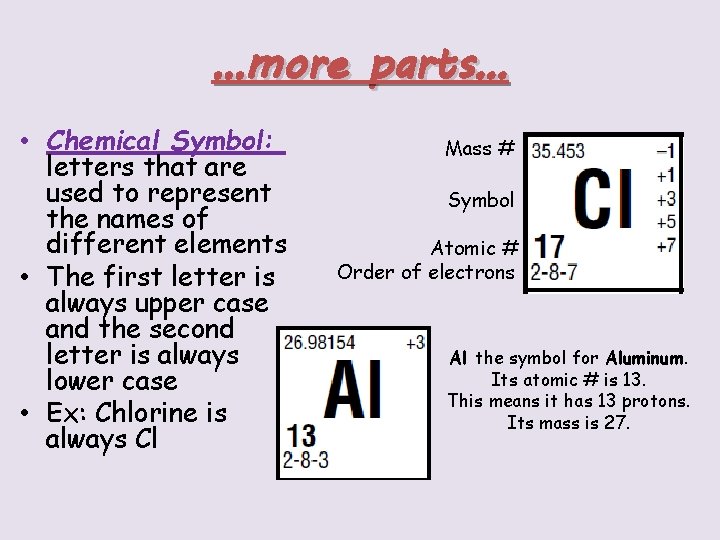
…more parts… • Chemical Symbol: letters that are used to represent the names of different elements • The first letter is always upper case and the second letter is always lower case • Ex: Chlorine is always Cl Mass # Symbol Atomic # Order of electrons Al the symbol for Aluminum. Its atomic # is 13. This means it has 13 protons. Its mass is 27.
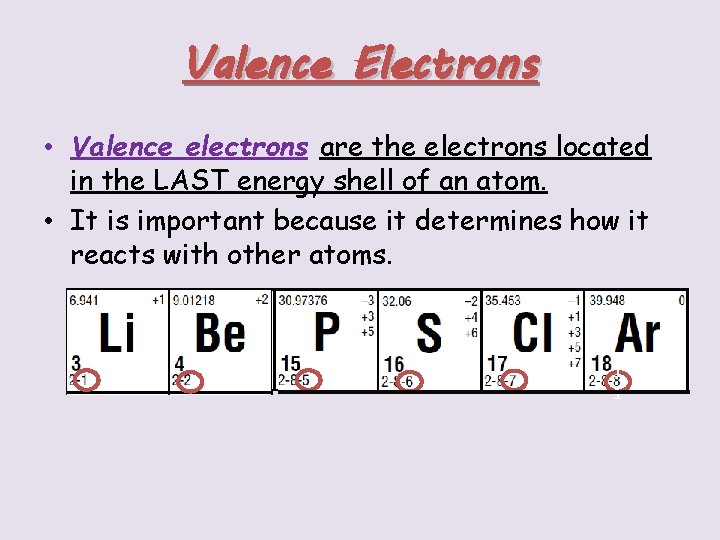
Valence Electrons • Valence electrons are the electrons located in the LAST energy shell of an atom. • It is important because it determines how it reacts with other atoms. 1 1
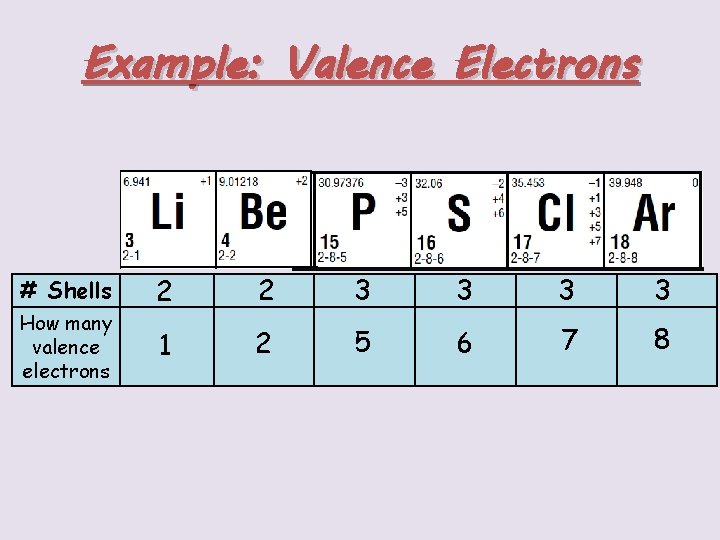
Example: Valence Electrons # Shells How many valence electrons 2 2 3 3 1 2 5 6 7 8

Families or Groups • There are columns on the periodic table called families or groups. • Each family/group is named and they tell us the number of valence electrons an atom has. • Just like our families, elements in the same family/group have a lot in common. – Group 17 are used as cleaning products
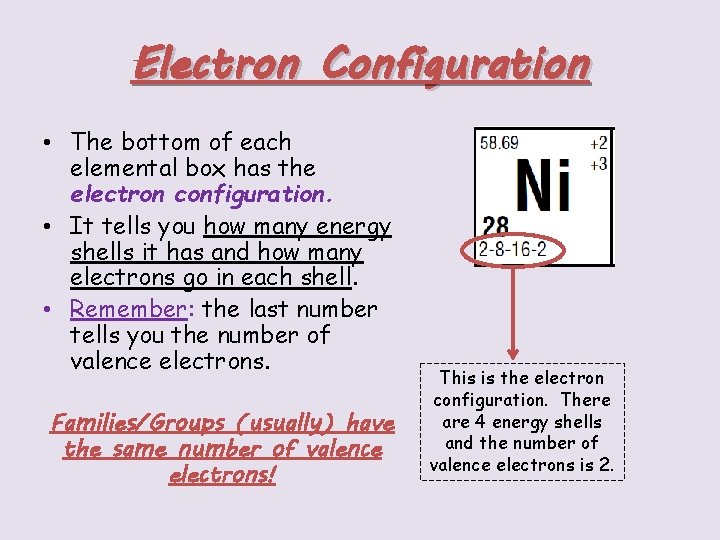
Electron Configuration • The bottom of each elemental box has the electron configuration. • It tells you how many energy shells it has and how many electrons go in each shell. • Remember: the last number tells you the number of valence electrons. Families/Groups (usually) have the same number of valence electrons! This is the electron configuration. There are 4 energy shells and the number of valence electrons is 2.
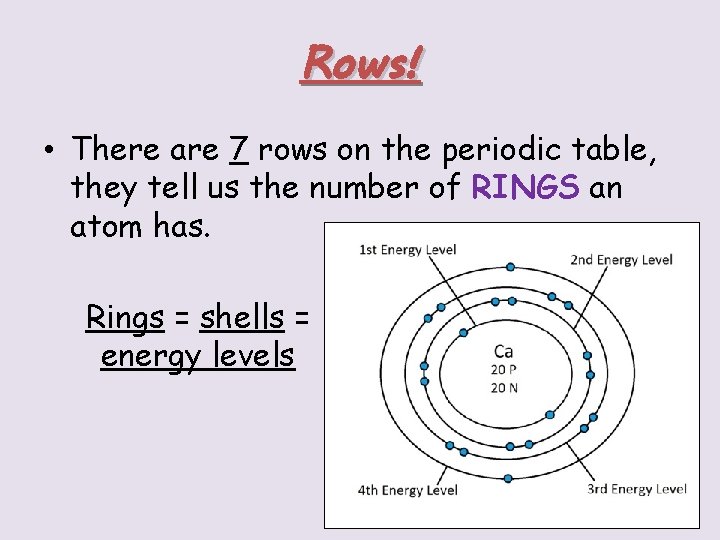
Rows! • There are 7 rows on the periodic table, they tell us the number of RINGS an atom has. Rings = shells = energy levels
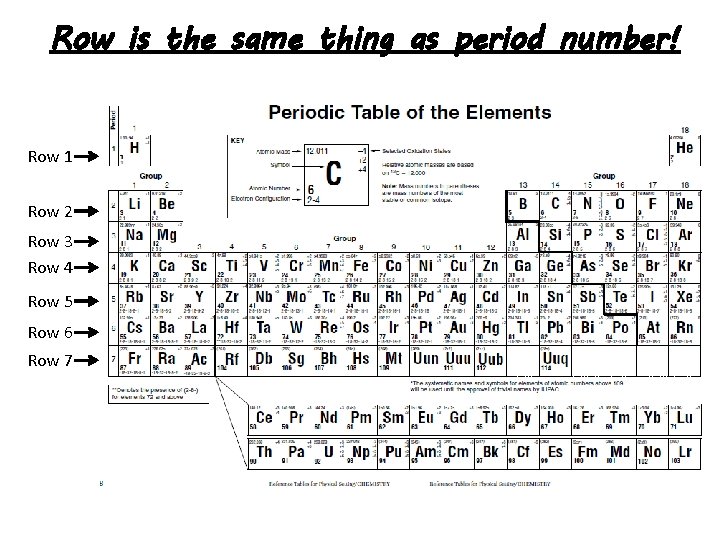
Row is the same thing as period number! Row 1 Row 2 Row 3 Row 4 Row 5 Row 6 Row 7
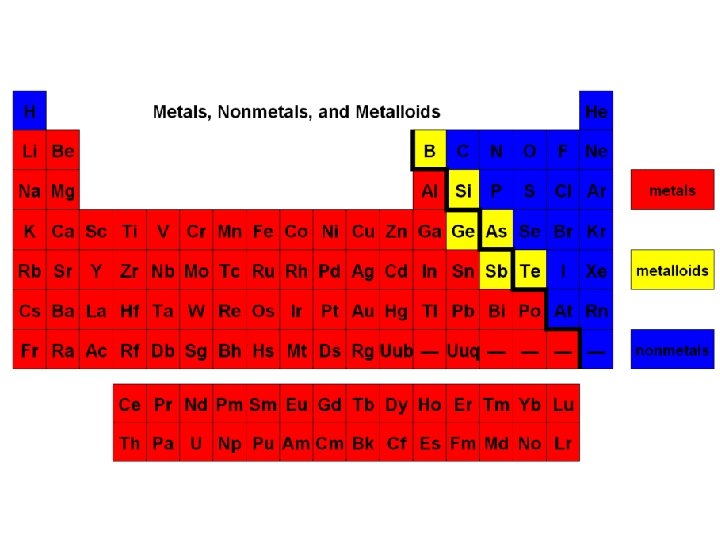

Metals • Metals constitute most of the Periodic Table. • They are on the LEFT of the staircase Properties of metals: 1. Malleable (can be hammered) 2. Ductile (can be drawn into a wire) 3. Has luster (shiny) 4. Good conductors of heat and electricity

Nonmetals • Nonmetals are located to the RIGHT of the staircase. Properties of nonmetals: 1. Dull 2. Poor conductors of heat and electricity 3. A lot of nonmetals are gasses at room temperature 4. NOT malleable and NOT ductile 5. Brittle (easily smashed into a powder) SULFUR

Metalloids • Metalloids are in between metals and nonmetals and are on the staircase. Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), and Tellurium (Te) • Properties of metalloids: 1. They are semiconductors 2. They have some properties of metals 3. They have some properties of nonmetals Ex: Te is shiny AND brittle Antimony

Family 1 / Group 1 • These are called the Alkali Metals. • Highly reactive, shiny, color of silver, and very soft • Since they are in Group 1, they have 1 valence electron.

Family 2 / Group 2 • These are called the Alkaline Earth Metals. • Since they are in Family 2, they have 2 valence electrons. • They are reactive metals, the color of silver, and are denser than group 1.
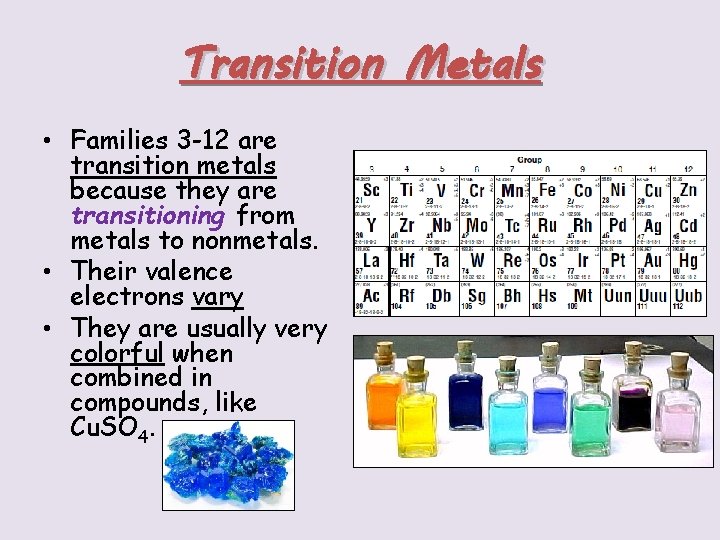
Transition Metals • Families 3 -12 are transition metals because they are transitioning from metals to nonmetals. • Their valence electrons vary • They are usually very colorful when combined in compounds, like Cu. SO 4.
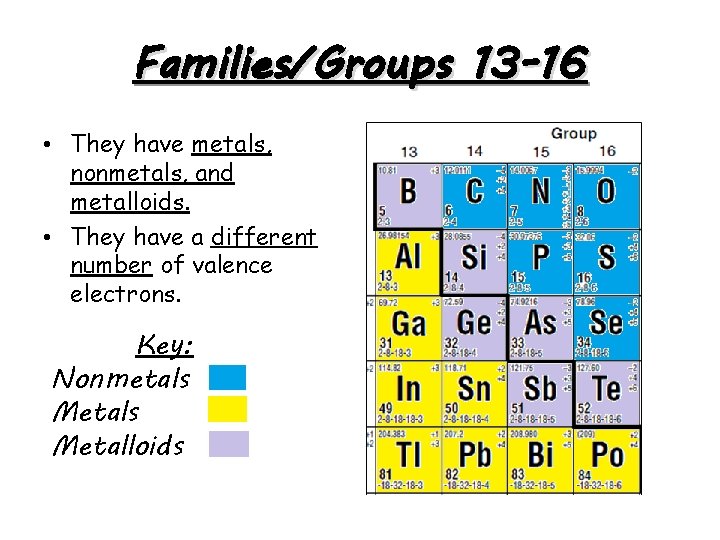
Families/Groups 13 -16 • They have metals, nonmetals, and metalloids. • They have a different number of valence electrons. Key: Nonmetals Metalloids

Family 17 / Group 17 • These are called the Halogens. • They are all nonmetals. • They are very reactive and are poor conductors of electricity • They usually combine with group 1 to make salts F

Family 18 / Group 18 • These are called the Noble Gasses. • They are all nonmetals • They are unreactive, colorless, odorless gasses at room temperature.

The extra 2 periods • The extra 2 rows are called the Lanthanides and Actinides and are there for a reason! – If they were placed where they belong they would push the elements in the respective rows/periods OUT of the correct columns! – Also, their placement would put them in Transition Metals; which means their location is more or less irrelevant, while the placement of elements in Families/Groups 13 -18 is significant. Lanthanides Actinides
- Slides: 24