The Periodic Table Lesson 2 the Atom Science

The Periodic Table Lesson 2 - the Atom Science Chemistry - Key Stage 3 Miss Willett 1

What do you remember from last time? 1. What is an element? 1. Where are non-metals on the periodic table? 1. What is the name of the line that separates metals and non-metals? 2

Quick fire question round! Which is smaller, an atom or a proton? What do you find in the nucleus of an atom? Where do we find electrons? 3
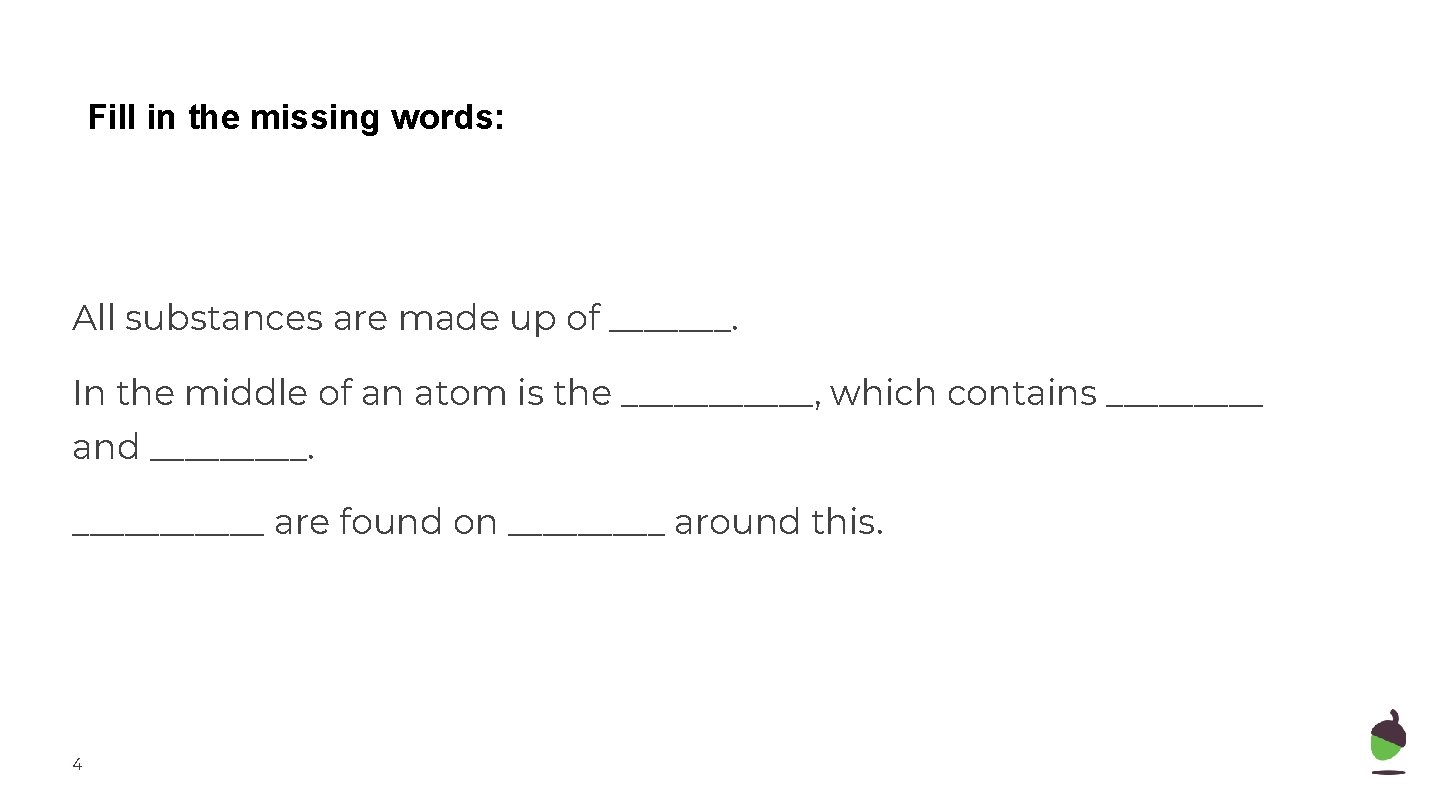
Fill in the missing words: All substances are made up of _______. In the middle of an atom is the ______, which contains _____ and ______ are found on _____ around this. 4

True or false? Electrons are always positively charged You can represent the nucleus of an atom as a circle Protons and electrons have the same mass 5

Q 1) Draw an atom, and label the subatomic particles Q 2) Add information about the subatomic particles 6
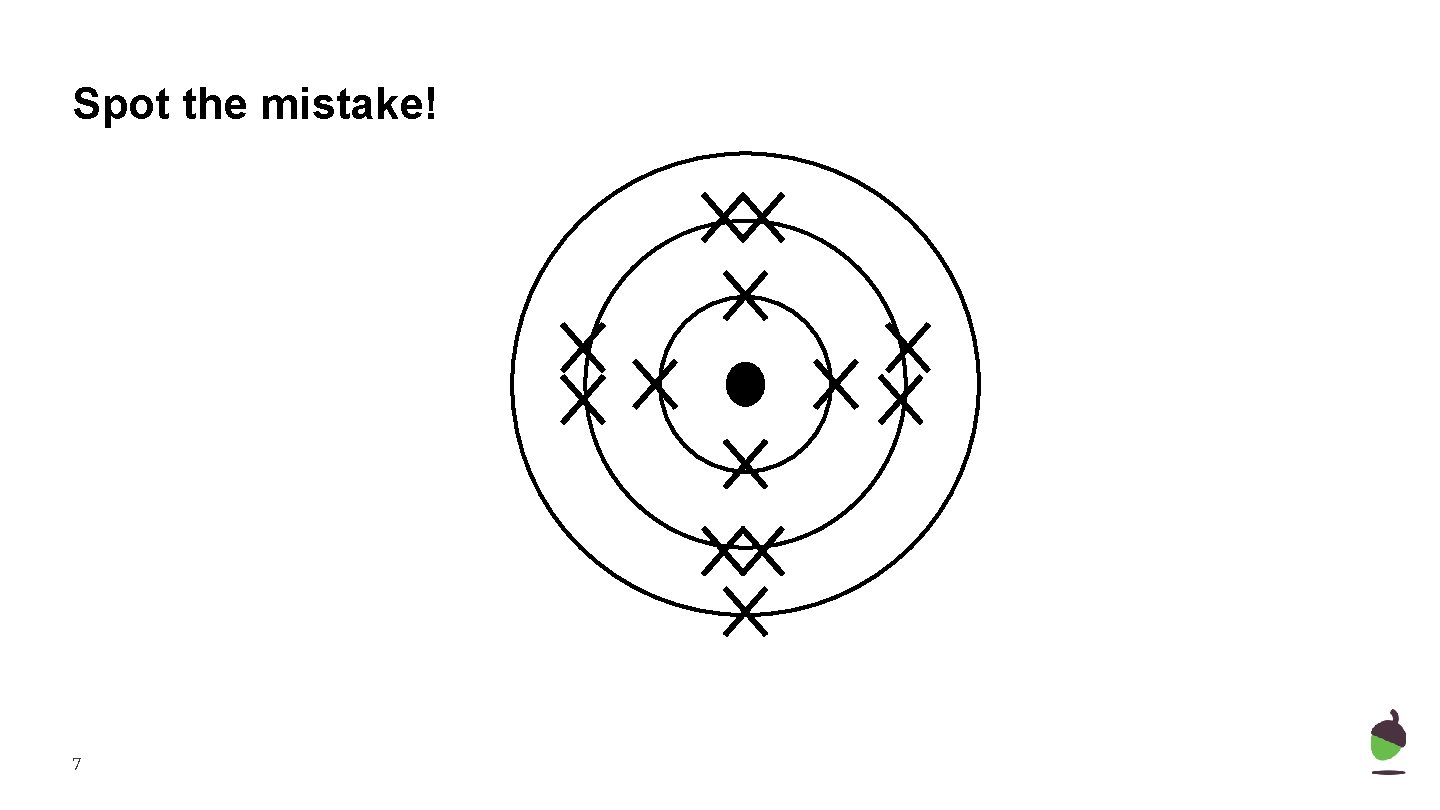
Spot the mistake! 7
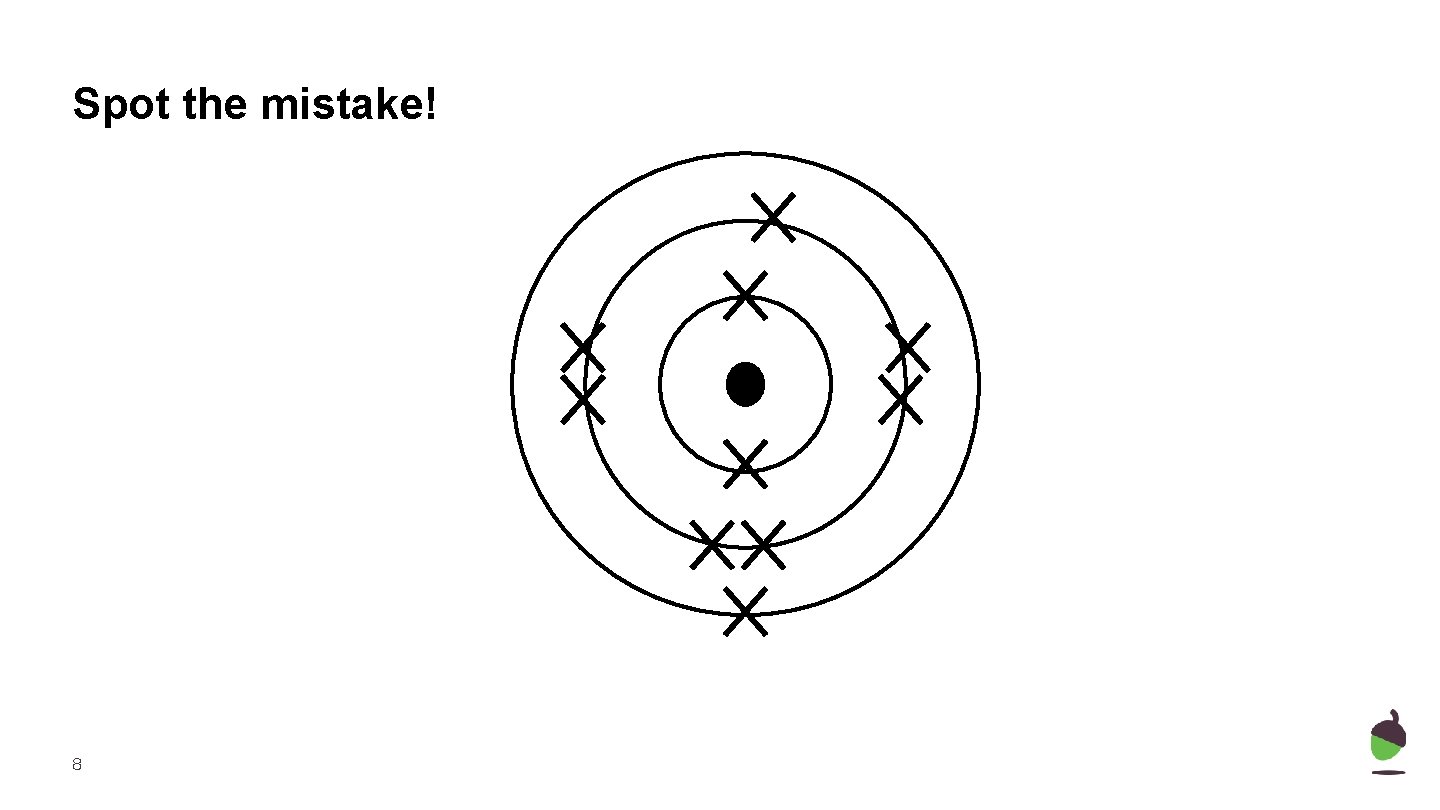
Spot the mistake! 8
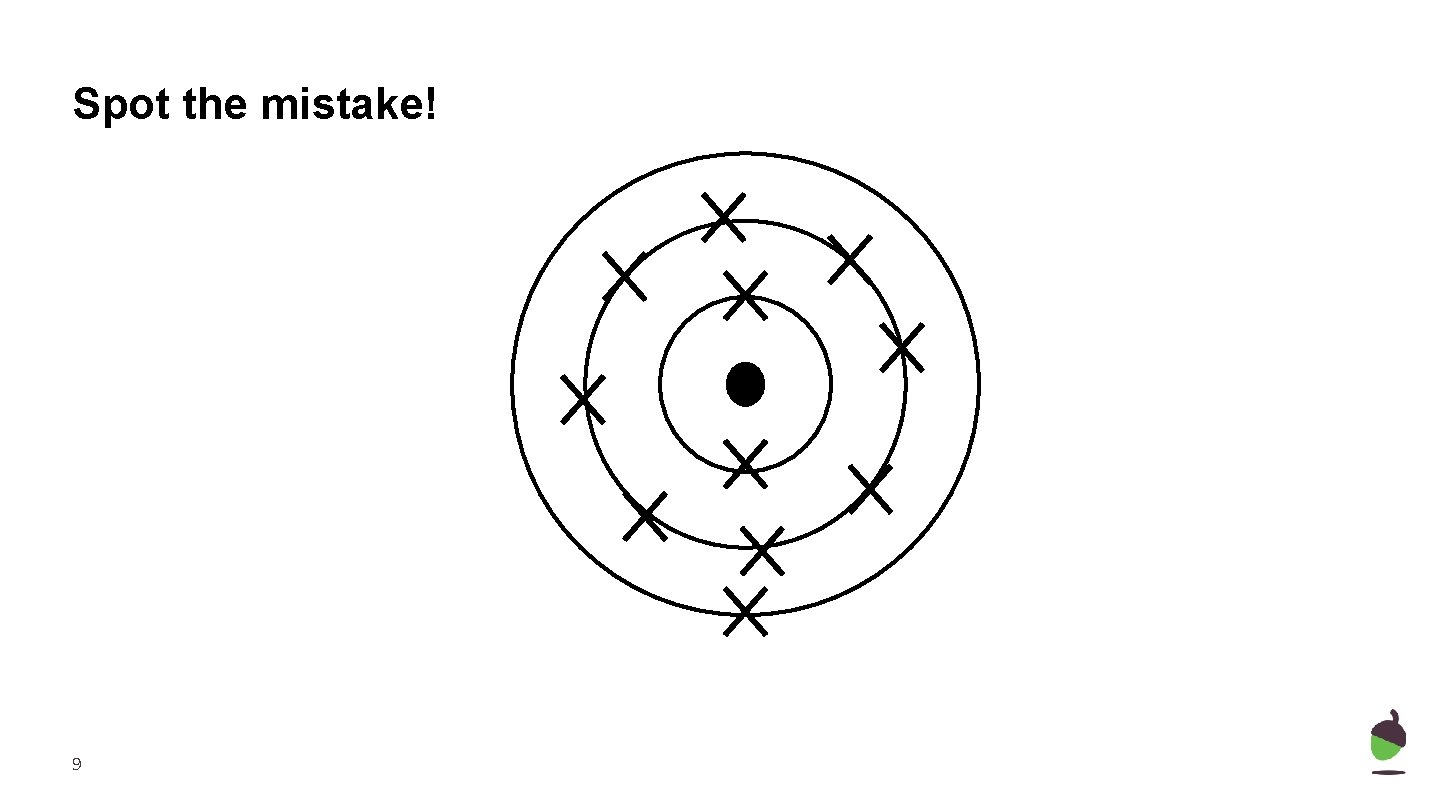
Spot the mistake! 9
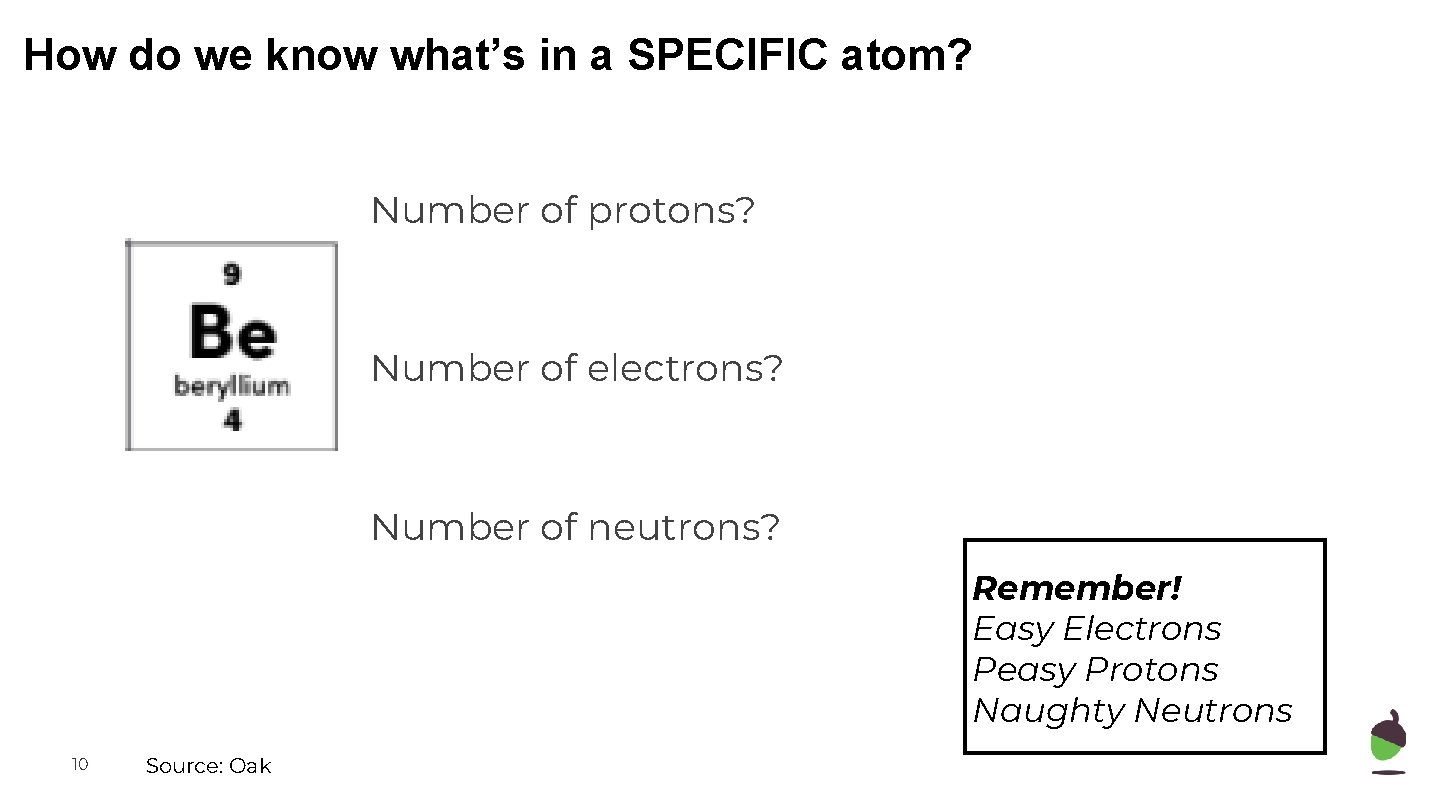
How do we know what’s in a SPECIFIC atom? Number of protons? Number of electrons? Number of neutrons? Remember! Easy Electrons Peasy Protons Naughty Neutrons 10 Source: Oak
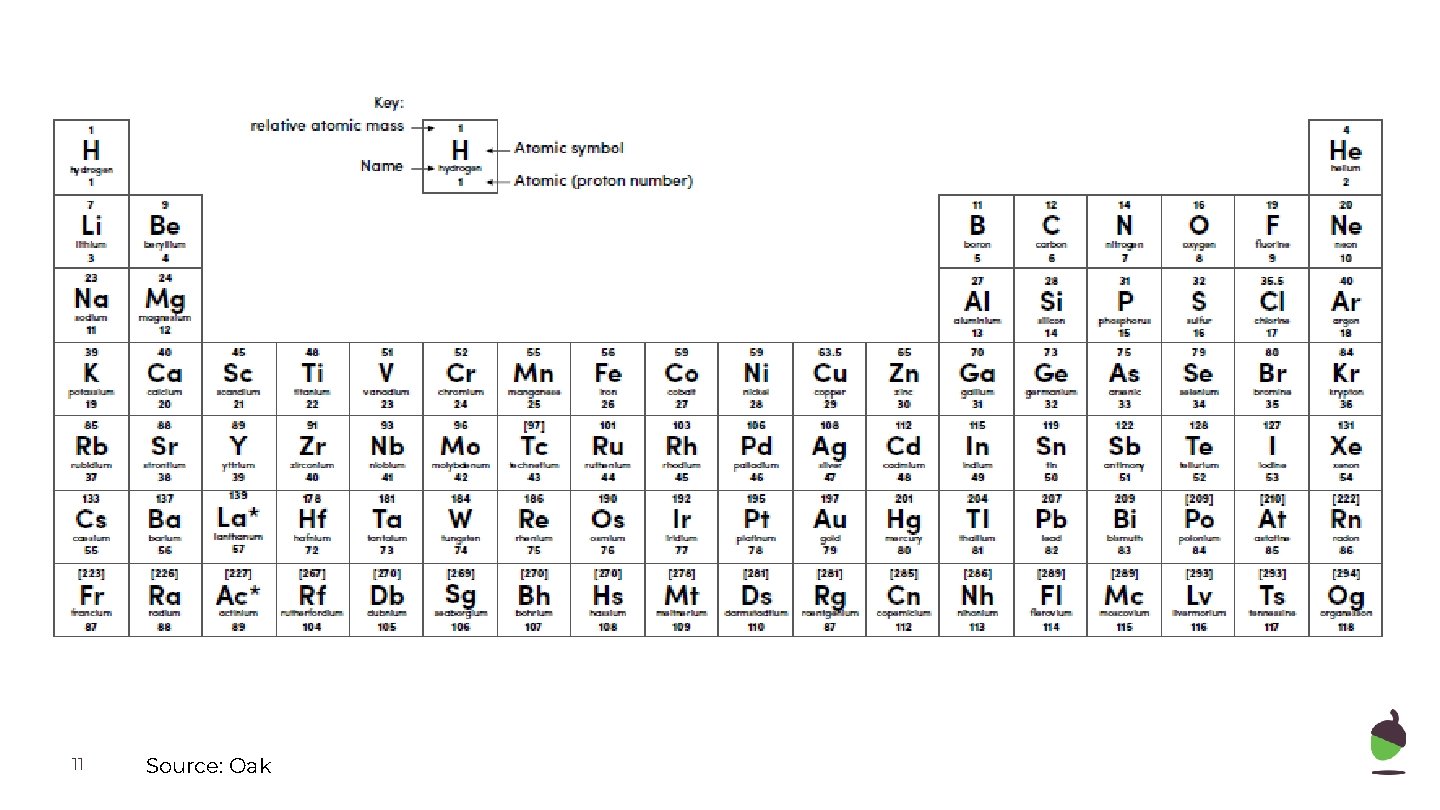
11 Source: Oak
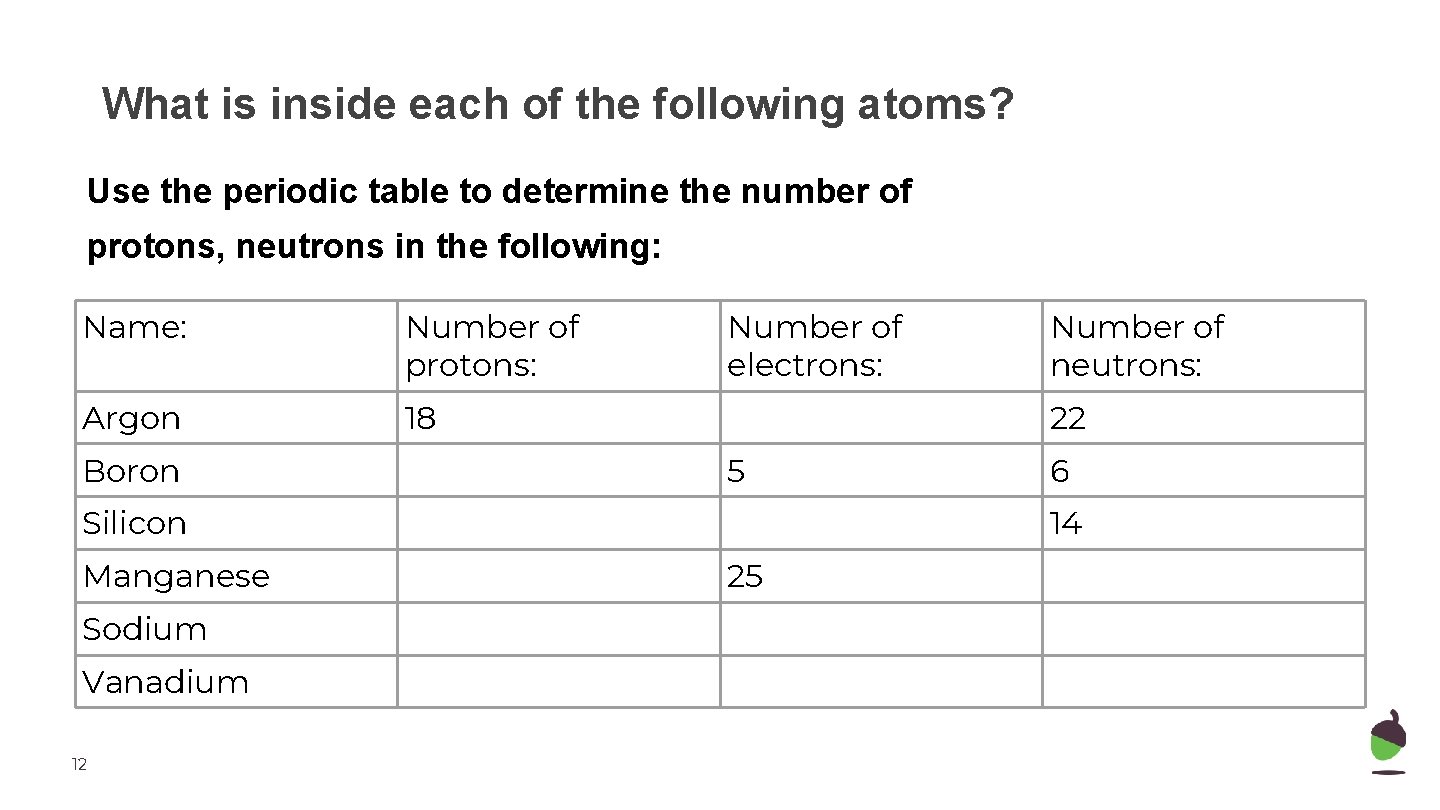
What is inside each of the following atoms? Use the periodic table to determine the number of protons, neutrons in the following: Name: Number of protons: Argon 18 Boron Number of electrons: 22 5 Silicon Manganese Sodium Vanadium 12 Number of neutrons: 6 14 25

Bringing it all together. . ● Draw the correct electronic configuration for Aluminium ● Describe its atomic structure 13 Source: Oak
- Slides: 13