The Periodic Table Learning Objectives All will recall
The Periodic Table Learning Objectives All will recall that the Periodic Table was constructed by Dimitri Mendeleev Most will understand that he built his ideas on the work of earlier scientists. Some will be able to explain that the table orders the elements according to their chemical properties.

Democritus (460 BC t 0 370 BC) Democritus was a Greek philosopher. He came up with many ideas and thoughts. In particular he provided the idea that all matter is composed of tiny, indivisible particles. He called these particles ‘atomos’ which means ‘indivisible’ in Greek. Of course there was no way to prove this theory and so it was left unchanged for many centuries.
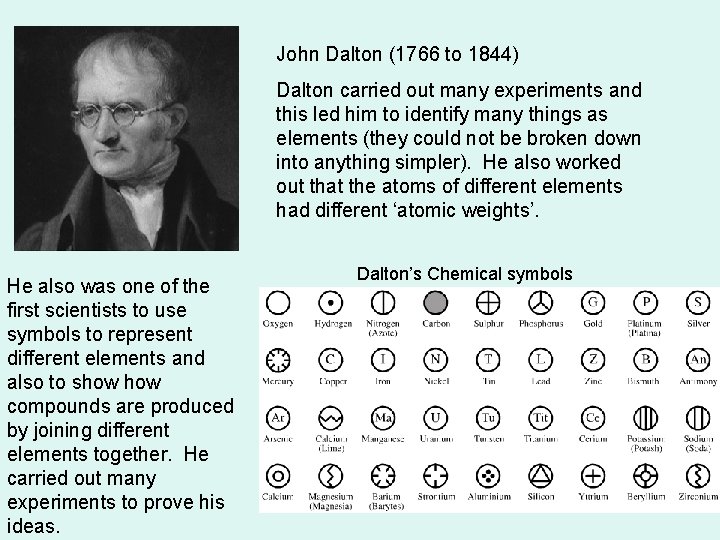
John Dalton (1766 to 1844) Dalton carried out many experiments and this led him to identify many things as elements (they could not be broken down into anything simpler). He also worked out that the atoms of different elements had different ‘atomic weights’. He also was one of the first scientists to use symbols to represent different elements and also to show compounds are produced by joining different elements together. He carried out many experiments to prove his ideas. Dalton’s Chemical symbols

Johann Wolfgang Döbereiner (1780 to 1849) Dobereiner carried out many investigations using the known elements. He noticed that some elements could be grouped together because they had similar physical or chemical properties. Several of these groups had three members. He also noticed that in some of these groups the atomic weight of one of the elements was the average (approximately) of the other two atomic weights. He called these groups ‘triads’ but he couldn’t get enough information to take the links further. Lithium Ar = 7. Potassium Ar = 40. Sodium Ar = 23 7+40 = 23. 5 2 Chlorine Ar = 35. 5, Iodine Ar = 127, Bromine Ar = 80 127 + 35. 5 = 81. 25 2

John Newlands (1837 to 1898) Newlands was an English Chemist who worked for a sugar refining company. In his spare time he investigated the properties of the known elements. He built upon the work of earlier scientists and came up with a table of elements where they were listed in increasing atomic weight. He noticed that there seemed to be a pattern in his table where elements with similar properties ended up in the same column. However these matches didn’t work all of the time and no matter how many times he re-drew it he could never get everything to match up.

Dmitri Mendeleev (1834 to 1907) Mendeleev was a Russian Chemist. He was also trying to spot a pattern in the elements. He knew the work of Dalton, Dobereiner and Newlands as well as other scientists. Mendeleev knew that there was a pattern to it all and that he would find it if he looked hard enough. He studied all the known elements and wrote down their chemical properties on small cards. He then tried to spot patterns by laying the cards out on a table and sorting them into groups and families. He spent years carrying out experiments and trying to get his pack of cards to make sense. It is said ( It may be just a story) that one night he dreamed of his pack of cards laid out on his table in a specific pattern. He woke from his dream, grabbed the cards and laid them out in the way he had dreamed about them! Suddenly it all made sense! Watch the video ‘Periodic table of elements’
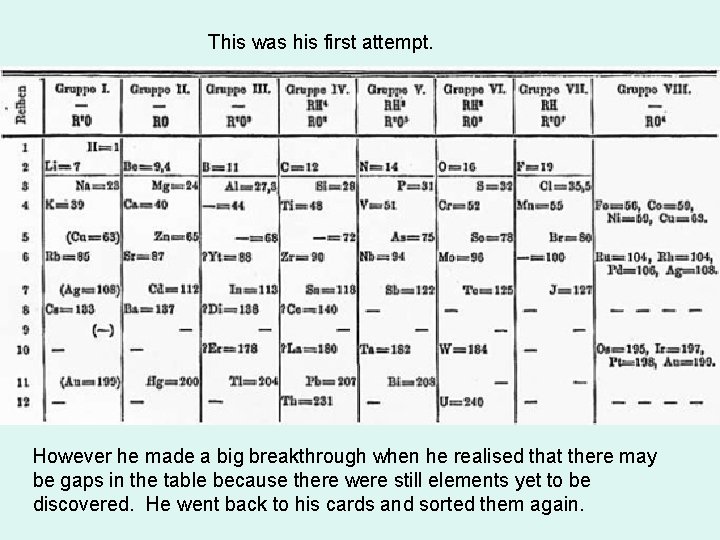
This was his first attempt. However he made a big breakthrough when he realised that there may be gaps in the table because there were still elements yet to be discovered. He went back to his cards and sorted them again.
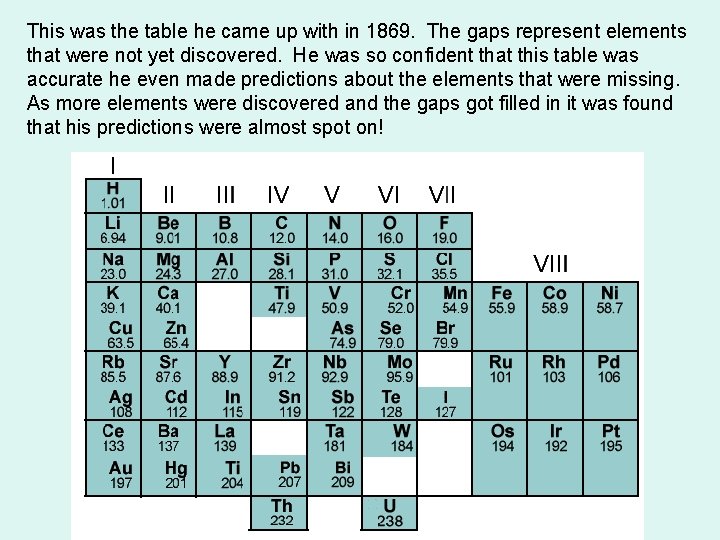
This was the table he came up with in 1869. The gaps represent elements that were not yet discovered. He was so confident that this table was accurate he even made predictions about the elements that were missing. As more elements were discovered and the gaps got filled in it was found that his predictions were almost spot on!
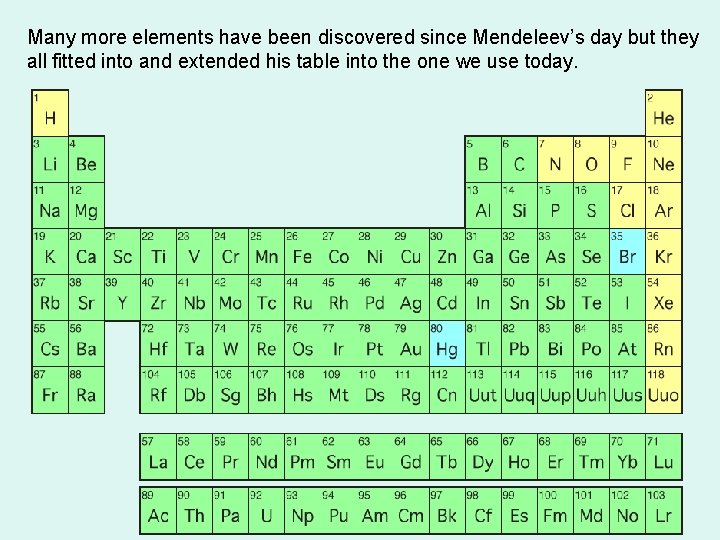
Many more elements have been discovered since Mendeleev’s day but they all fitted into and extended his table into the one we use today.
The Periodic Table Learning Objectives All will recall that the Periodic Table was constructed by Dimitri Mendeleev Most will understand that he built his ideas on the work of earlier scientists. Some will be able to explain that Mendeleev’s table orders the elements according to their chemical properties
- Slides: 10