The Periodic Table III Organization Metallic Character Rows
The Periodic Table III. Organization ¨Metallic Character ¨Rows & Columns ¨Table Sections

A. Metallic Character ¨ Metals ¨ Nonmetals ¨ Metalloids (color your table)

Characteristics of Metals A. Metals 1. 2. 3. 4. They are solid They are shiny Good conductors of electricity They are ductile a. Can be rolled into thin wires 5. They are malleable a. Hammered into thin sheets 6. They lose electrons easily

Characteristics of Nonmetals A. Nonmetals 1. 2. 3. 4. 5. 6. 7. Are brittle (break easy) Are not shiny Is an invisible gas Not malleable Not ductile Poor conductors of electricity Tend to gain electrons

Characteristics of Metalloids A. Metalloids 1. Have characteristics of both metals and nonmetals a. Some are shiny, some are dull b. Are semiconductors a. Conduct electricity but not well c. Not as brittle as nonmetals
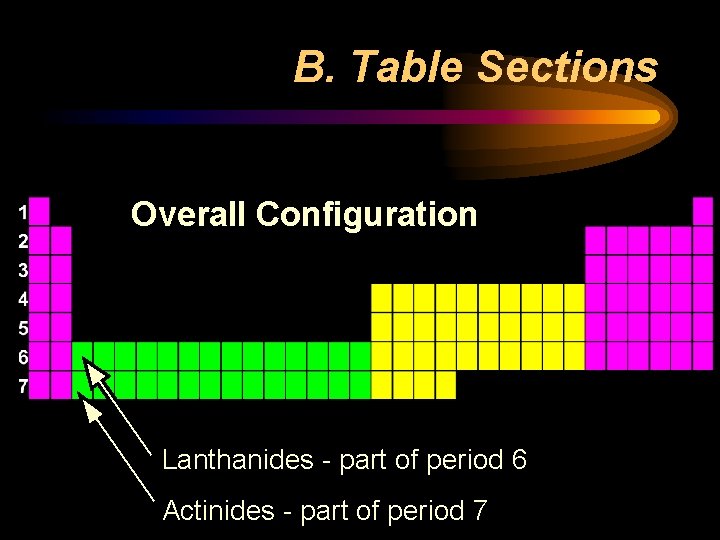
B. Table Sections Overall Configuration Lanthanides - part of period 6 Actinides - part of period 7
C. Columns & Rows ¨ Group (Family) ¨ Period (label your table)

Practice Problems A. Determine if the following elements are classified as a metal, nonmetal, or metalloid. 1. Chlorine 2. Zinc 3. Lithium 4. Lanthanum 5. Bromine 6. Boron 7. Mercury 8. Astatine

Practice Problems A. Match up the elements that are in the same family. Neon Fluorine Hydrogen Oxygen Cesium Thallium Silicon Carbon Strontium Iodine Beryllium Radon Aluminum Selenium

Practice Problems A. Match up the elements that are in the same period. Ununbium Lithium Nitrogen Francium Hydrogen Bismuth Sulfur Helium Sodium Xenon Bromine Barium Molybdenum Calcium
- Slides: 10