The Periodic Table How is the Periodic Table

The Periodic Table
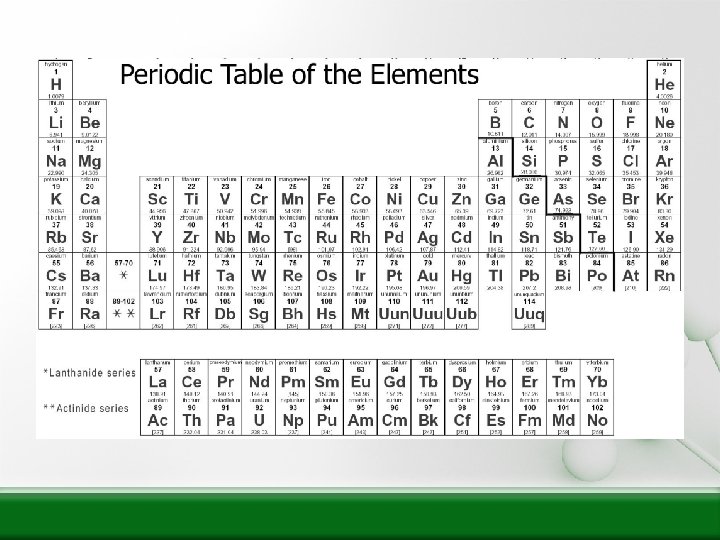

How is the Periodic Table Arranged? • In order of increasing atomic number in specific columns and rows.
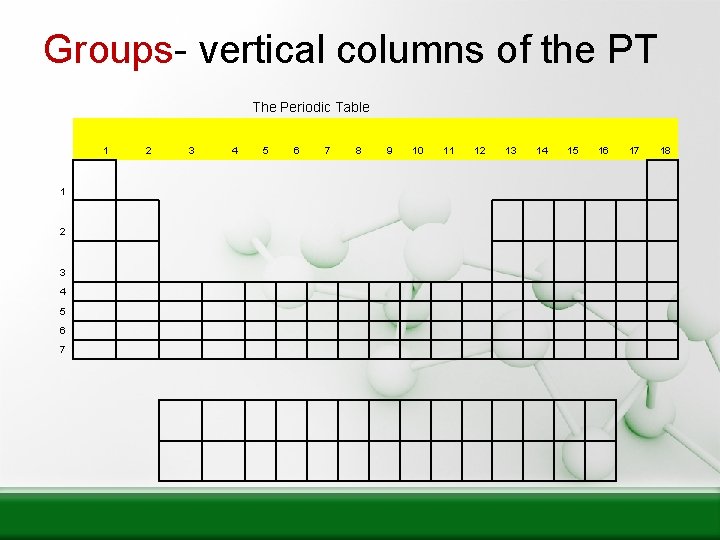
Groups- vertical columns of the PT The Periodic Table 1 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
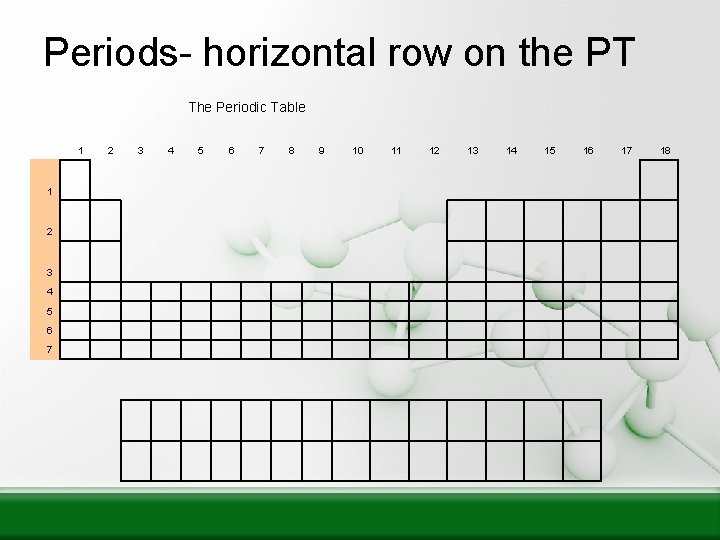
Periods- horizontal row on the PT The Periodic Table 1 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
Groups are important on the PT • Why? –The elements in a group have similar chemical and physical properties!
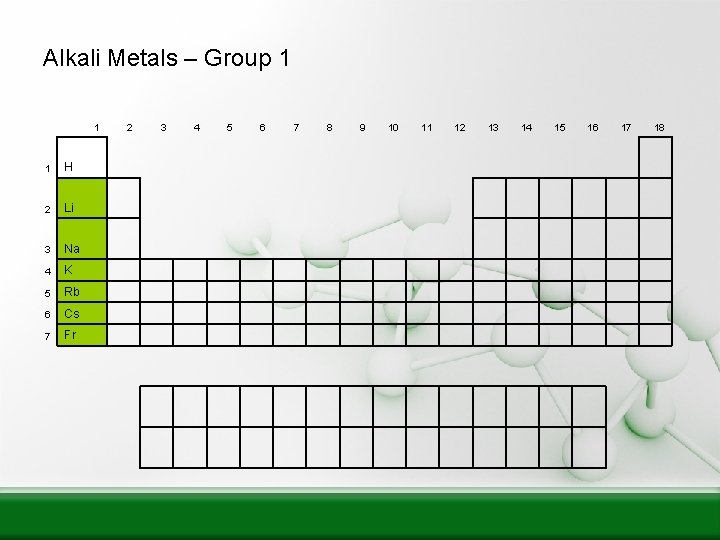
Alkali Metals – Group 1 1 1 H 2 Li 3 Na 4 K 5 Rb 6 Cs 7 Fr 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
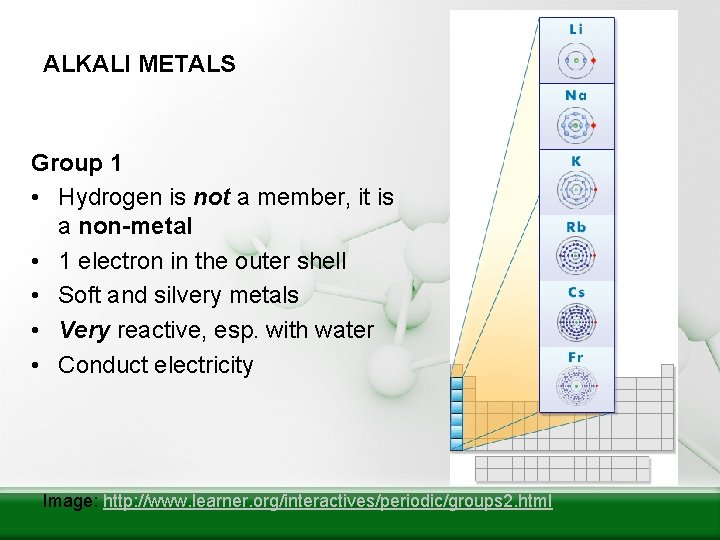
ALKALI METALS Group 1 • Hydrogen is not a member, it is a non-metal • 1 electron in the outer shell • Soft and silvery metals • Very reactive, esp. with water • Conduct electricity Image: http: //www. learner. org/interactives/periodic/groups 2. html
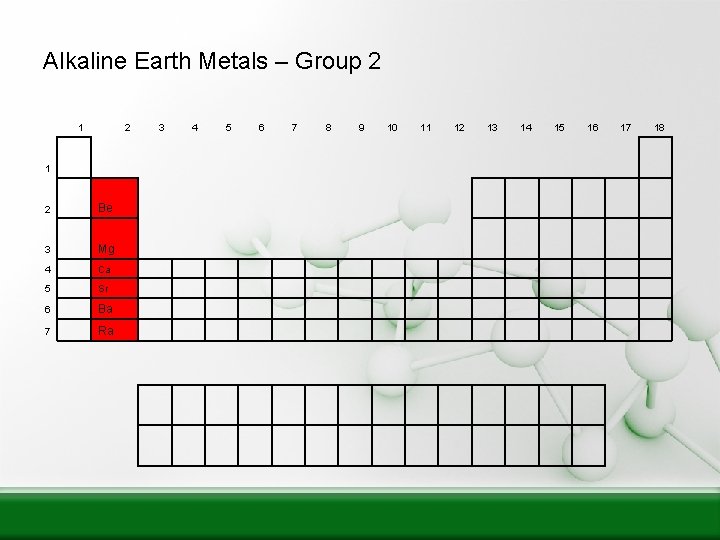
Alkaline Earth Metals – Group 2 1 2 Be 3 Mg 4 Ca 5 Sr 6 Ba 7 Ra 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
ALKALINE EARTH METALS Group 2 • 2 electrons in the outer shell • White and malleable • Reactive, but less than Alkali metals • Conduct electricity
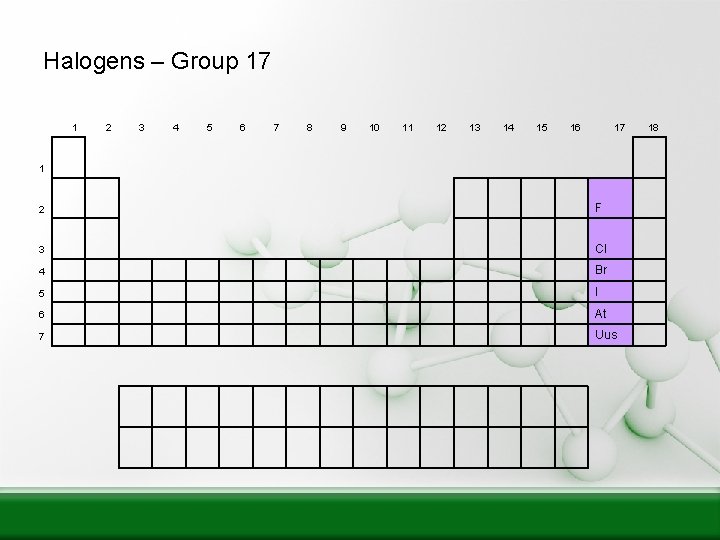
Halogens – Group 17 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 1 2 F 3 Cl 4 Br 5 I 6 At 7 Uus 18
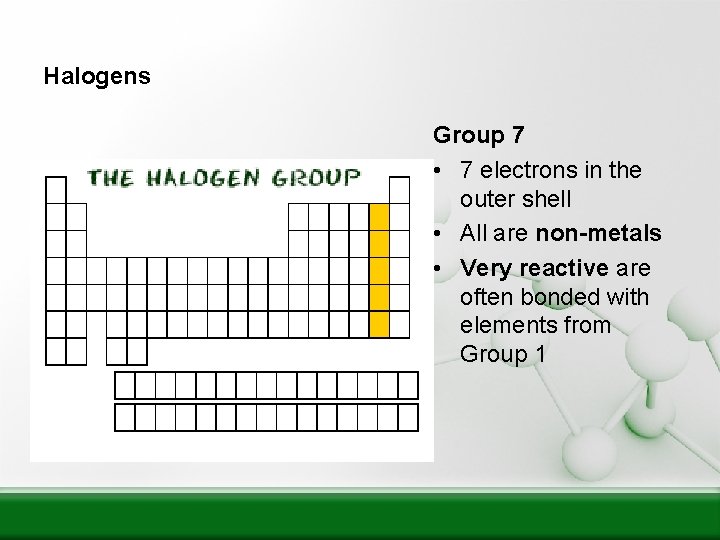
Halogens Group 7 • 7 electrons in the outer shell • All are non-metals • Very reactive are often bonded with elements from Group 1
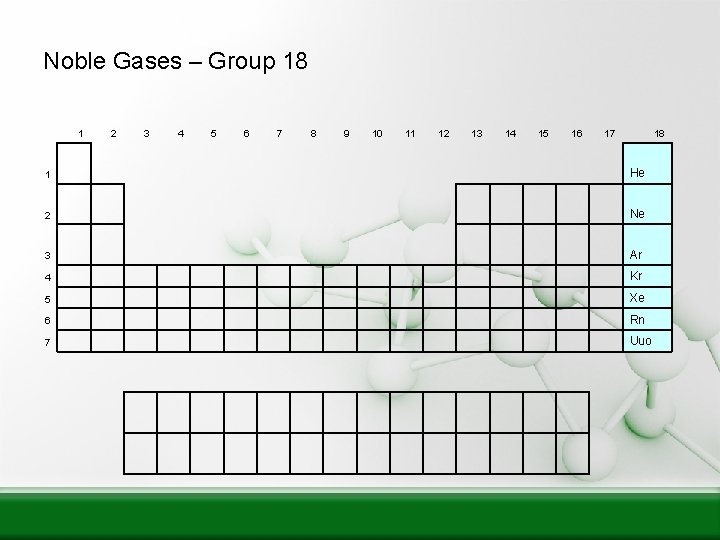
Noble Gases – Group 18 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 1 He 2 Ne 3 Ar 4 Kr 5 Xe 6 Rn 7 Uuo
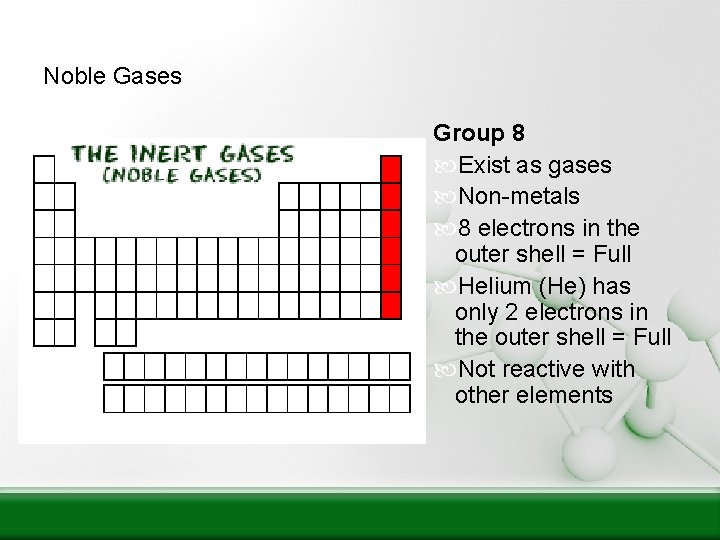
Noble Gases Group 8 Exist as gases Non-metals 8 electrons in the outer shell = Full Helium (He) has only 2 electrons in the outer shell = Full Not reactive with other elements
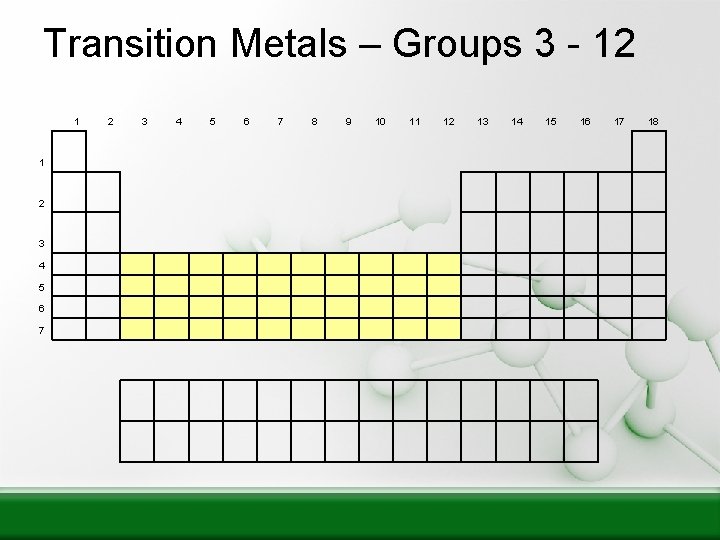
Transition Metals – Groups 3 - 12 1 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
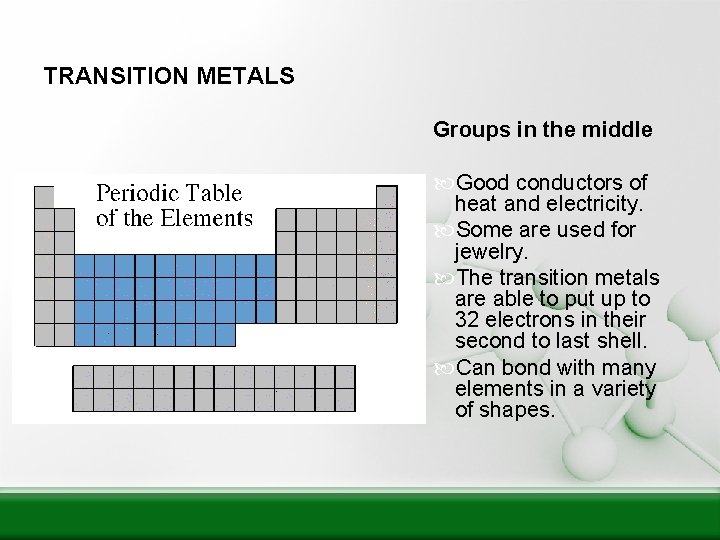
TRANSITION METALS Groups in the middle Good conductors of heat and electricity. Some are used for jewelry. The transition metals are able to put up to 32 electrons in their second to last shell. Can bond with many elements in a variety of shapes.
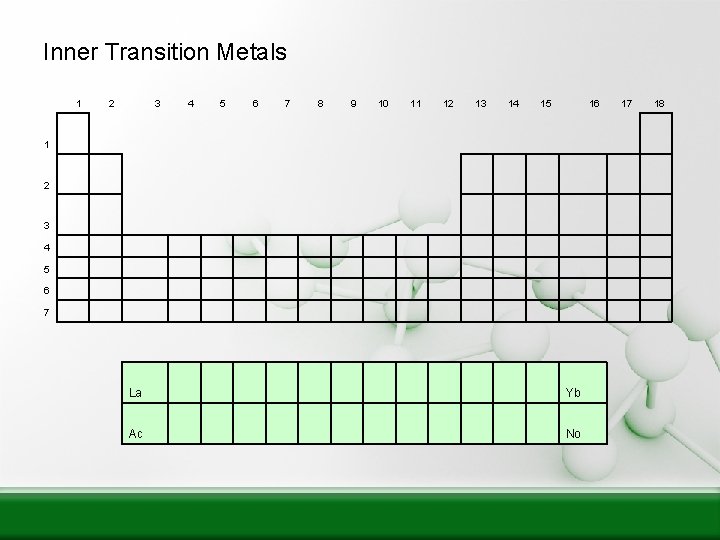
Inner Transition Metals 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 1 2 3 4 5 6 7 La Yb Ac No 17 18
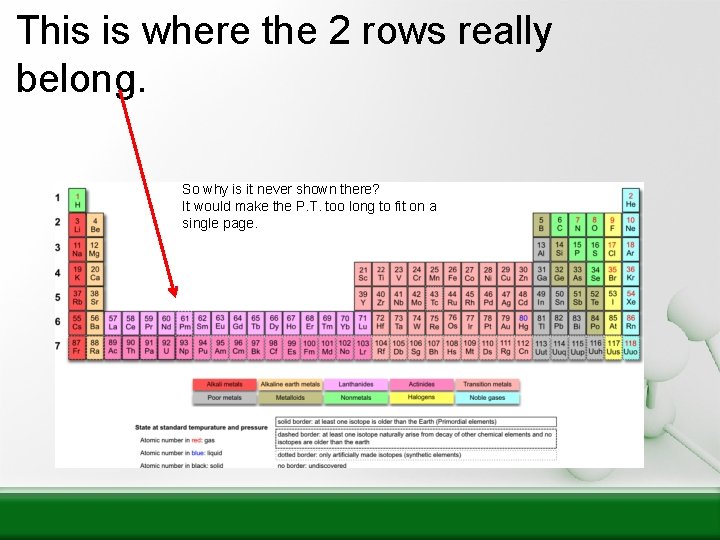
This is where the 2 rows really belong. So why is it never shown there? It would make the P. T. too long to fit on a single page.
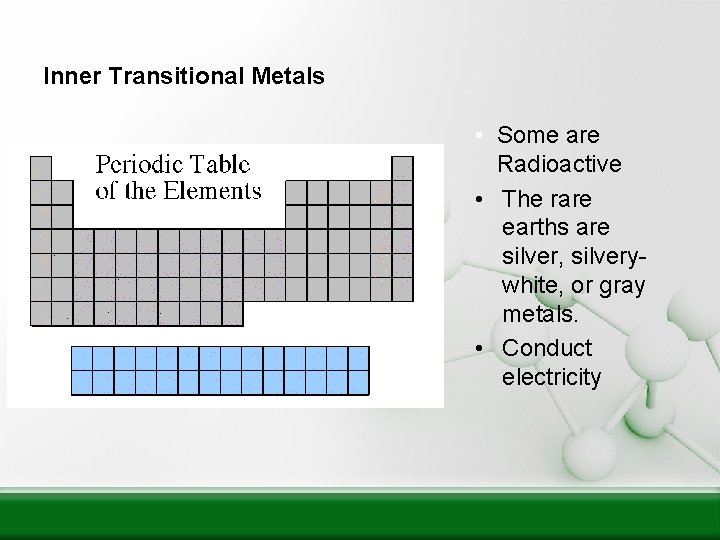
Inner Transitional Metals • Some are Radioactive • The rare earths are silver, silverywhite, or gray metals. • Conduct electricity

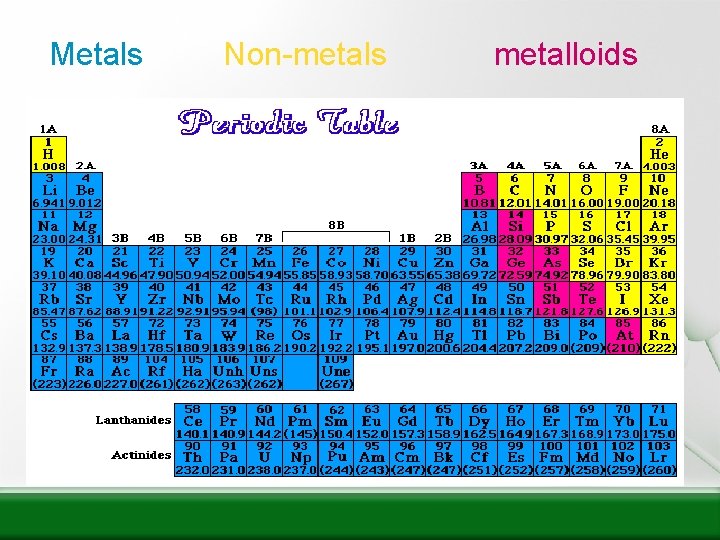
Metals • Lustrous • Good conductors of heat & electricity • Malleable – can be pounded into thin sheets • Ductile – can be drawn into thin wire
Metals Non-metals metalloids
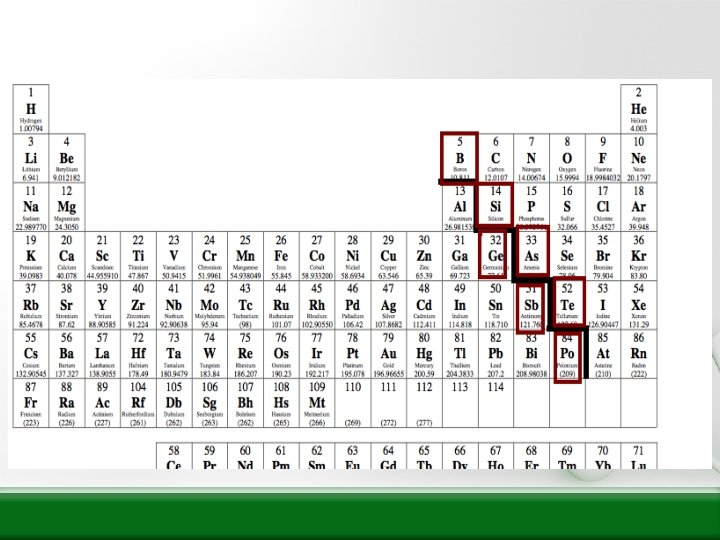
Metalloids
MMetalloids • Metalloids – Are between the metals and the nonmetals on the P. T. – Can have properties of both metals and nonmetals – There are 7 METALLOIDS • Boron • Silicon • Germanium • Arsenic • Antimony • Tellurium • Polonium

Non-metals • Nonmetals – Are BRITTLE (shatter easily when hit) solids, or liquid or gas at room temperature – Are INSULATORS of heat and electricity – Solids are DULL S Cl
Diatomic Elements • Most elements can be isolated to atomic elements – individual atoms • 7 elements are too reactive to exist as individual atoms, instead, they are found as molecular elements – 2 atoms bonded together • • Hydrogen, H 2 Oxygen, O 2 Nitrogen, N 2 Chlorine, Cl 2 Bromine, Br 2 Iodine, I 2 Fluorine, F 2
- Slides: 25