The Periodic Table History Structure Trends Mendeleev 1870

The Periodic Table History Structure Trends
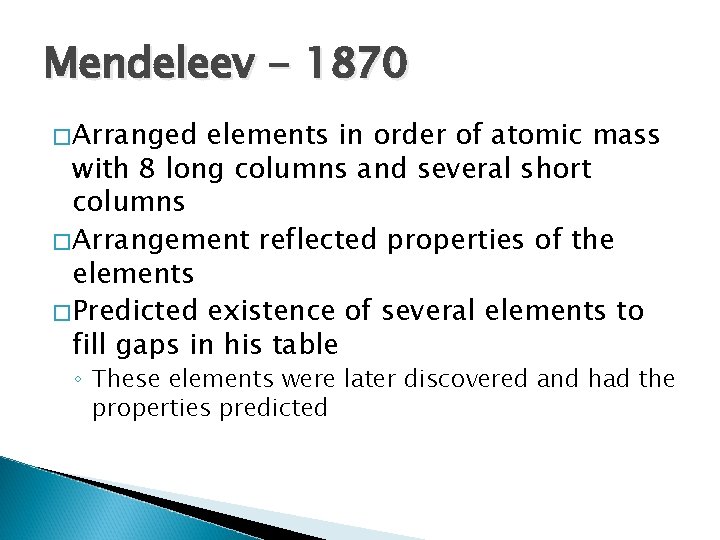
Mendeleev - 1870 �Arranged elements in order of atomic mass with 8 long columns and several short columns �Arrangement reflected properties of the elements �Predicted existence of several elements to fill gaps in his table ◦ These elements were later discovered and had the properties predicted

Mendeleev’s Periodic Law Properties of the elements are a periodic function of their atomic masses.
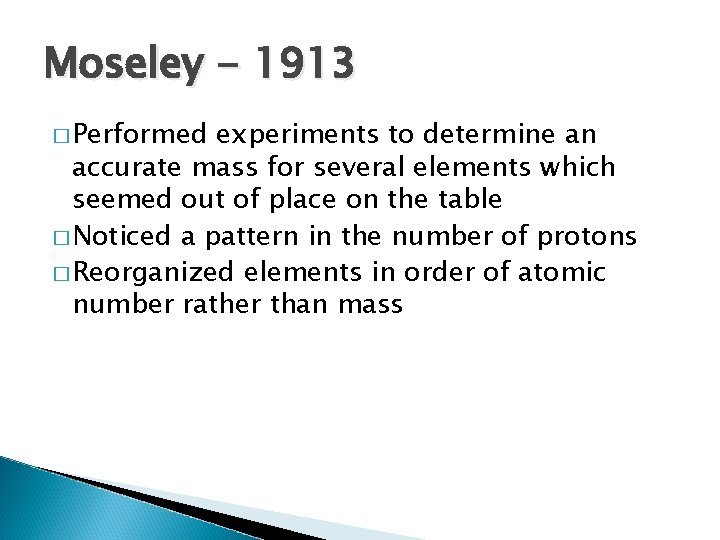
Moseley - 1913 � Performed experiments to determine an accurate mass for several elements which seemed out of place on the table � Noticed a pattern in the number of protons � Reorganized elements in order of atomic number rather than mass

Modern Periodic Law: Properties of elements are periodic functions of their atomic numbers.
Periods 1 7 rows 2 � 3 4 5 6 7
Groups or Families 1 18 2 13 14 15 16 17 3 4 5 6 7 8 9 10 11 12
This Arrangement Reflects: Properties � Increasing atomic number � Electron configuration �
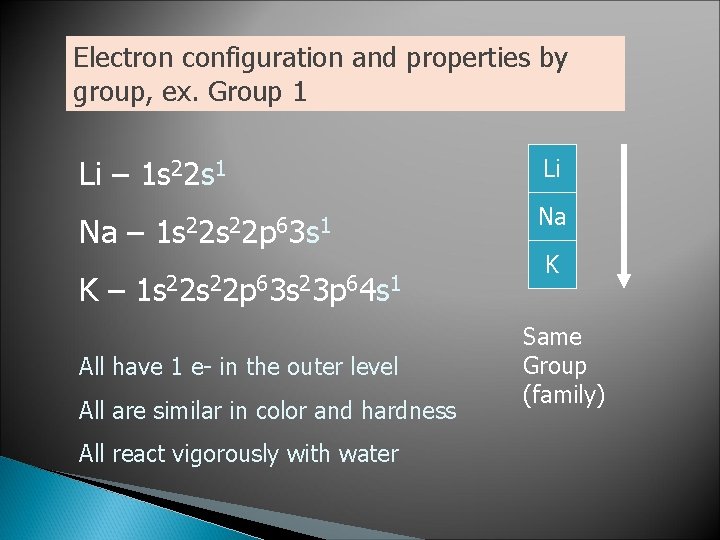
Electron configuration and properties by group, ex. Group 1 Li – 1 s 22 s 1 Na – 1 s 22 p 63 s 1 K – 1 s 22 p 63 s 23 p 64 s 1 All have 1 e- in the outer level All are similar in color and hardness All react vigorously with water Li Na K Same Group (family)
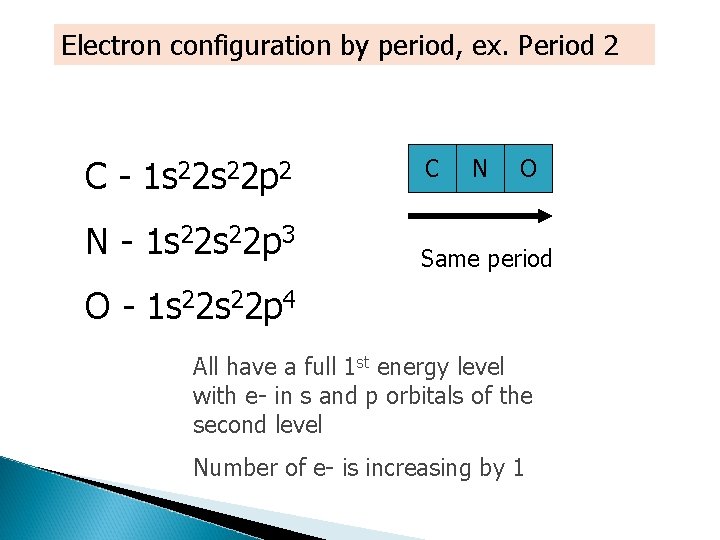
Electron configuration by period, ex. Period 2 C - 1 s 22 p 2 N - 1 s 22 p 3 C N O Same period O - 1 s 22 p 4 All have a full 1 st energy level with e- in s and p orbitals of the second level Number of e- is increasing by 1
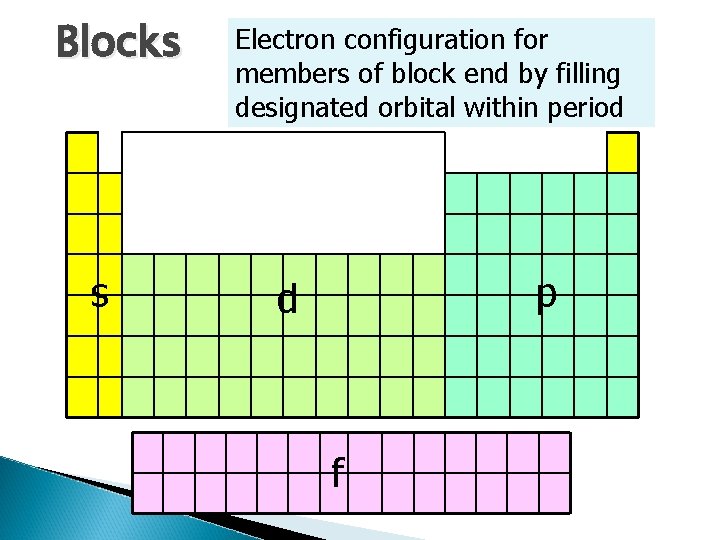
Blocks s Electron configuration for members of block end by filling designated orbital within period p d f
General Properties of Metals 1 to 3 e- in outer energy level of most � Lose e- to form + ions (cations) � Shiny (lustrous) � Hard � Malleable and ductile � Good conductors of heat and electricity � Solid at room temp (298 K), except Hg �

General Properties of Nonmetals: � � � 5 to 8 electrons in the outer level Gain e- to form – ions (anions) Brittle solids or gases Poor conductors of heat Poor conductors of electricity
Classification of Elements: Some elements have properties similar to both metals and nonmetals. � These are found bordering the stair-step dividing line. � ◦ Exception: Al is a metal � These elements are called metalloids
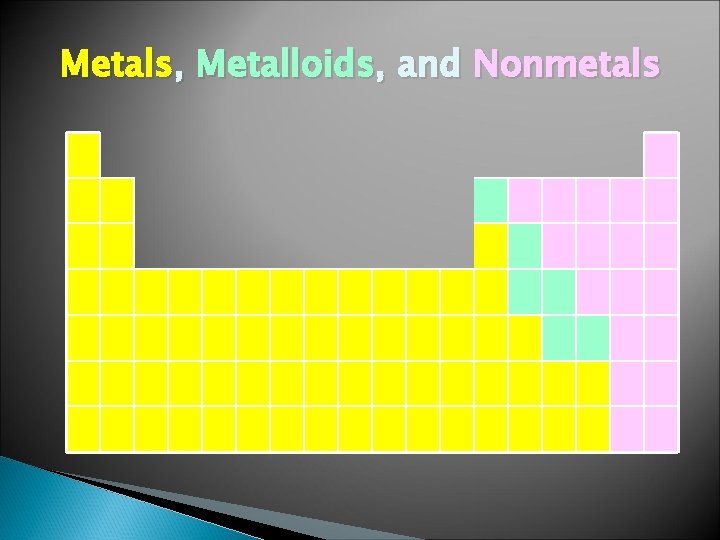
Metals, Metalloids, and Nonmetals

Some of the Families Have Special Names. Metals 1 – alkali metals 2 – alkaline earth metals 3 -12 – transition metals Elements 58 – 71 lanthanides Elements 90 – 103 actinides Non-metals 17 – halogens 18 – noble gases Rare Earth Metals
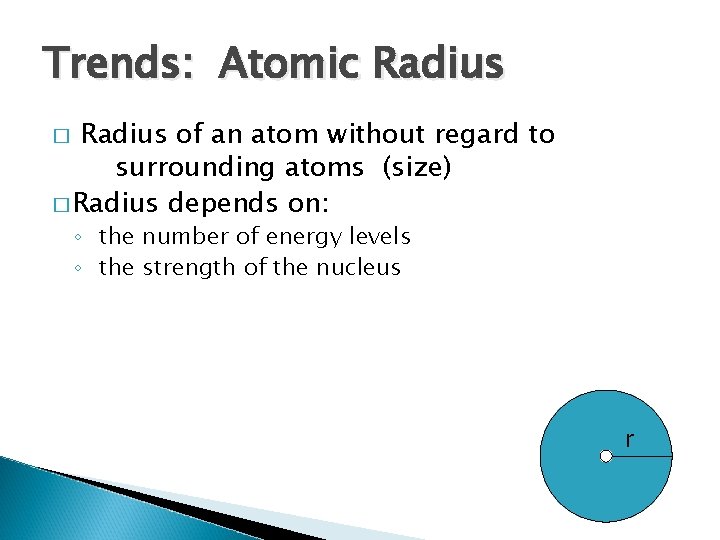
Trends: Atomic Radius of an atom without regard to surrounding atoms (size) � Radius depends on: � ◦ the number of energy levels ◦ the strength of the nucleus r
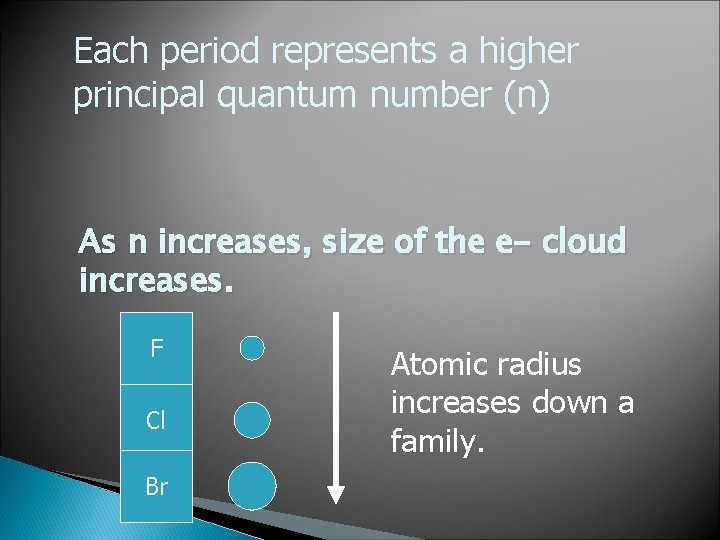
Each period represents a higher principal quantum number (n) As n increases, size of the e- cloud increases. F Cl Br Atomic radius increases down a family.

Across a period, nuclear charge increases by 1 for each element. A stronger nucleus acts like a stronger magnet which attracts the e- cloud. C N O Atomic radius decreases across a period.
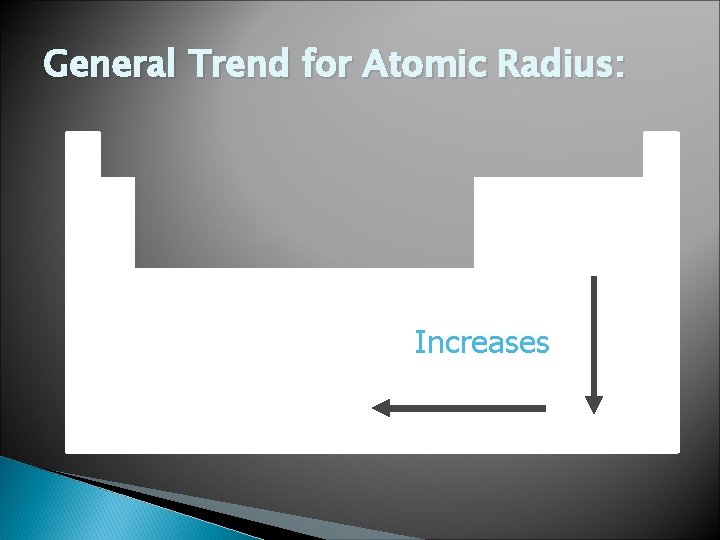
General Trend for Atomic Radius: Increases

Use position in the periodic table to determine which is larger? Na or Rb? Cl or I? Al or Si? K or Ca? Ag or Au? Ni or Cu? La or U? H or He?
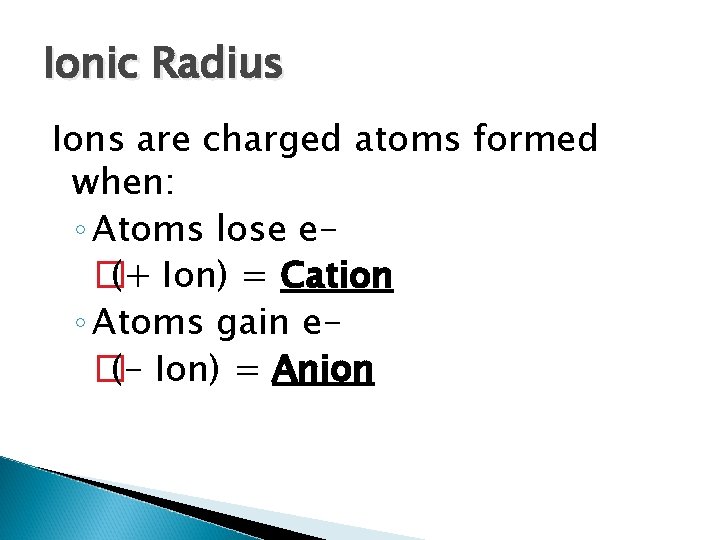
Ionic Radius Ions are charged atoms formed when: ◦ Atoms lose e� (+ Ion) = Cation ◦ Atoms gain e� (- Ion) = Anion
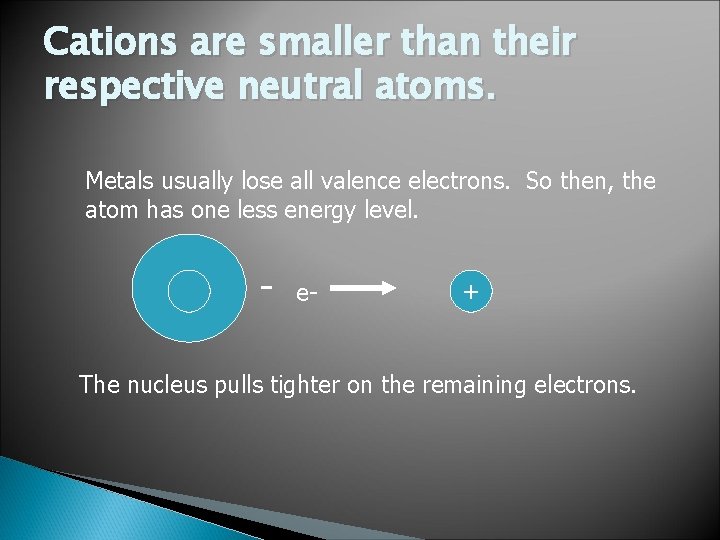
Cations are smaller than their respective neutral atoms. Metals usually lose all valence electrons. So then, the atom has one less energy level. - e- + The nucleus pulls tighter on the remaining electrons.
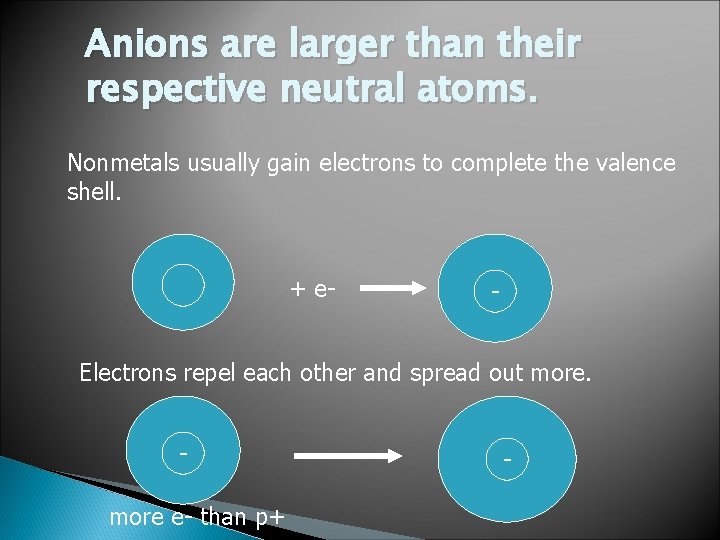
Anions are larger than their respective neutral atoms. Nonmetals usually gain electrons to complete the valence shell. + e- - Electrons repel each other and spread out more e- than p+ -

Which is larger? Ca or Ca+2 F or F– 1 K or K+1 O or O-2 F-, Ne or Na+

First Ionization Energy needed to remove the most loosely held electron from a gaseous atom. � Factors that affect ionization energy: � ◦ ◦ Radius Nuclear charge Shielding effect Stability of sublevels
Radius: � The greater the distance between the nucleus and the valence electrons, the easier it is to lose an electron.

Nuclear charge: # of p+ � Within a period, the higher the nuclear charge, the higher the ionization energy.
Shielding Effect: � Other e- block the pull of the nucleus on the outer e-. � Electrons repel each other.
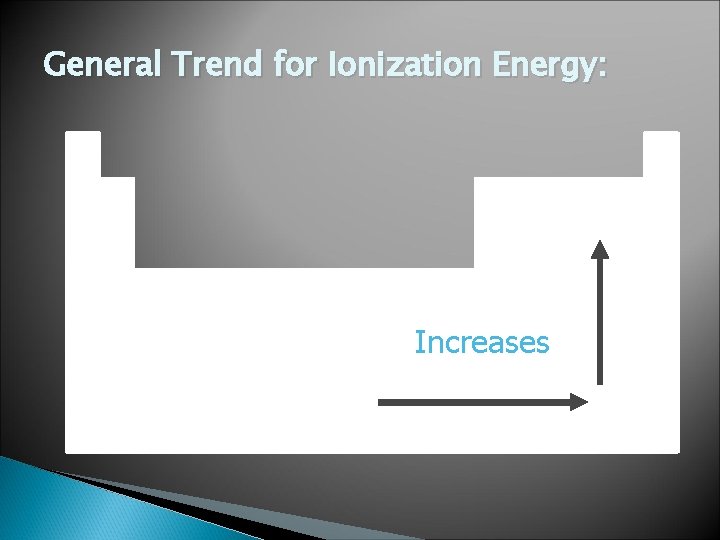
General Trend for Ionization Energy: Increases
Electron Affinity: Attraction of an atom for an additional electron. � Factors affecting EA: � ◦ ◦ Size Nuclear charge Shielding effect Stability of sublevels
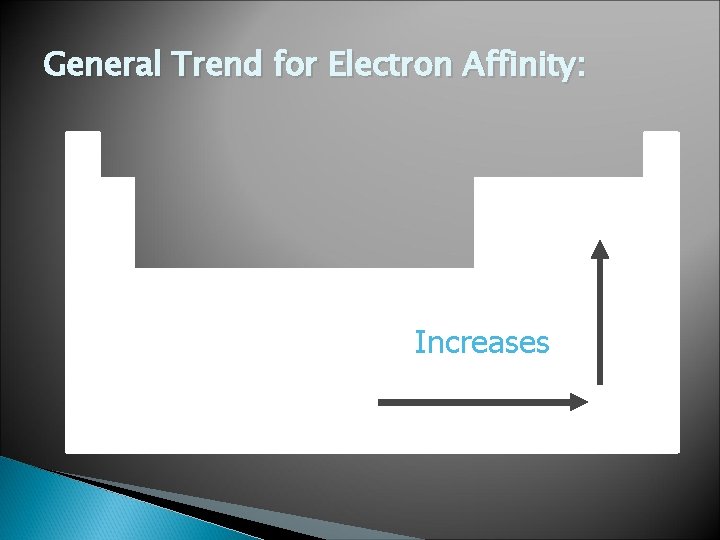
General Trend for Electron Affinity: Increases
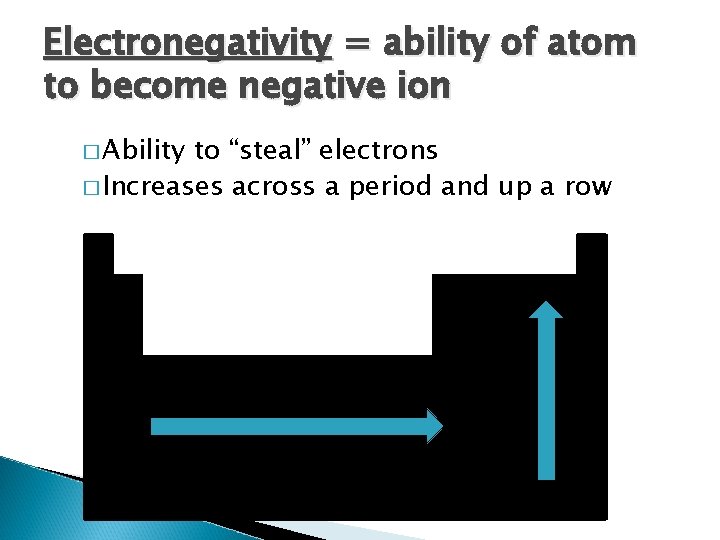
Electronegativity = ability of atom to become negative ion � Ability to “steal” electrons � Increases across a period and up a row
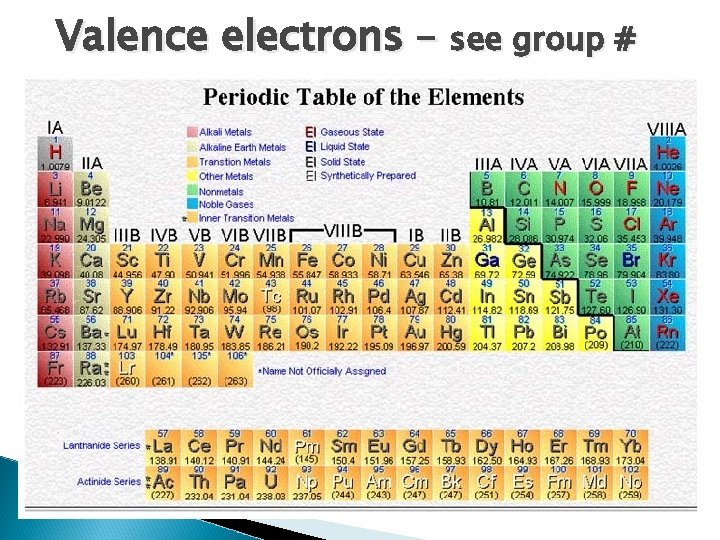
Valence electrons – see group #
- Slides: 34