The Periodic Table Group or Family goes top
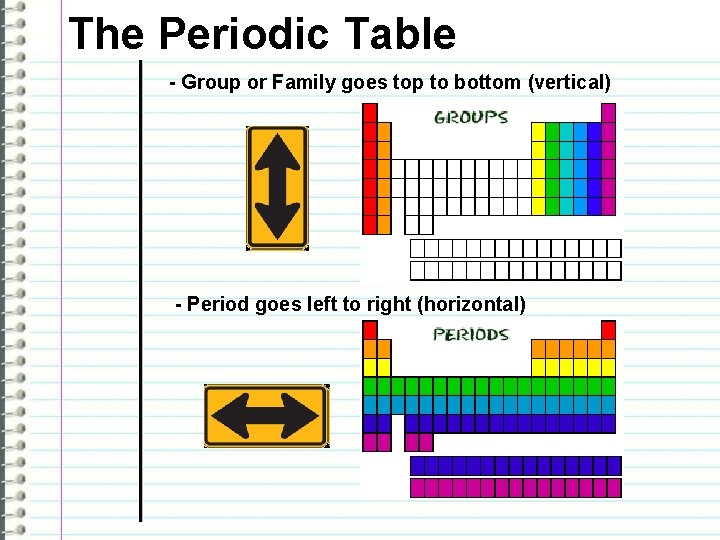
The Periodic Table - Group or Family goes top to bottom (vertical) - Period goes left to right (horizontal)
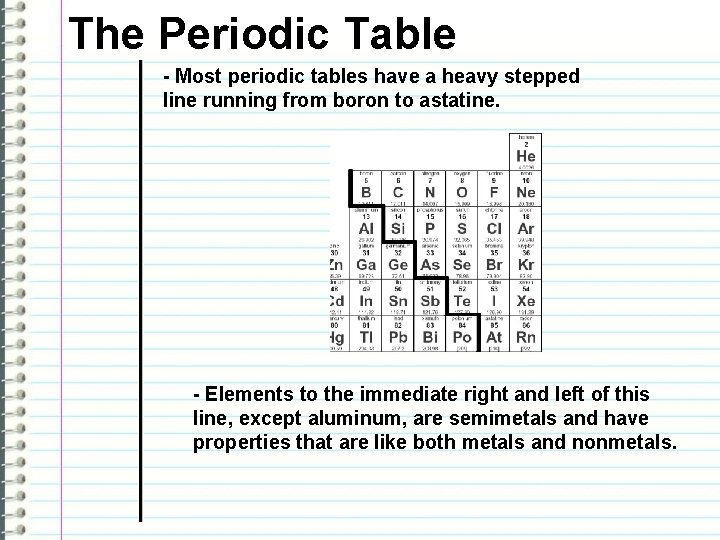
The Periodic Table - Most periodic tables have a heavy stepped line running from boron to astatine. - Elements to the immediate right and left of this line, except aluminum, are semimetals and have properties that are like both metals and nonmetals.
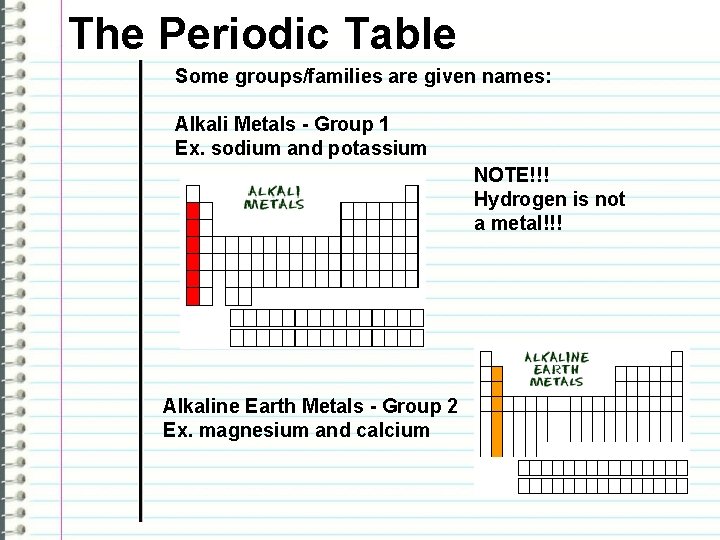
The Periodic Table Some groups/families are given names: Alkali Metals - Group 1 Ex. sodium and potassium NOTE!!! Hydrogen is not a metal!!! Alkaline Earth Metals - Group 2 Ex. magnesium and calcium
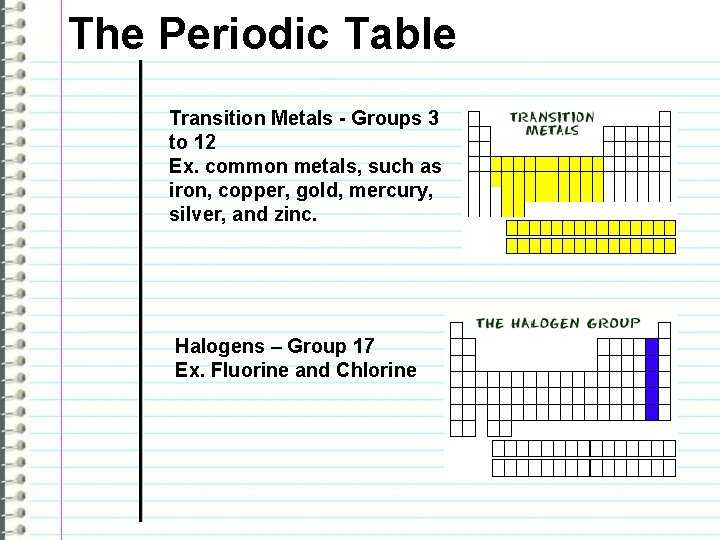
The Periodic Table Transition Metals - Groups 3 to 12 Ex. common metals, such as iron, copper, gold, mercury, silver, and zinc. Halogens – Group 17 Ex. Fluorine and Chlorine
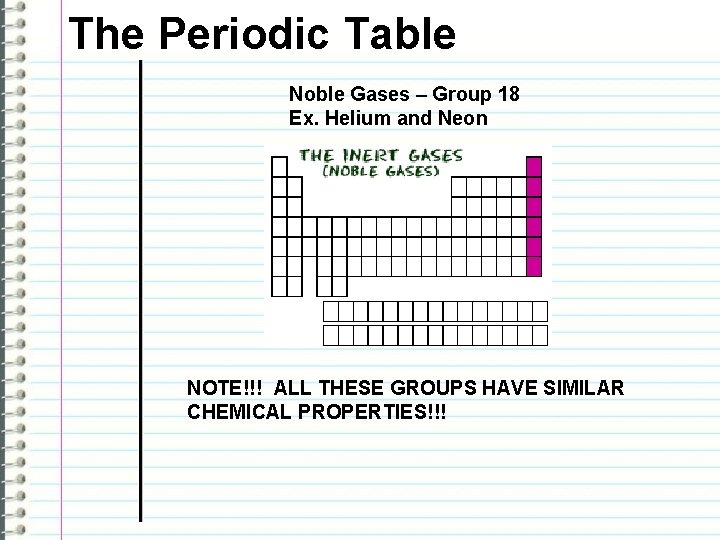
The Periodic Table Noble Gases – Group 18 Ex. Helium and Neon NOTE!!! ALL THESE GROUPS HAVE SIMILAR CHEMICAL PROPERTIES!!!
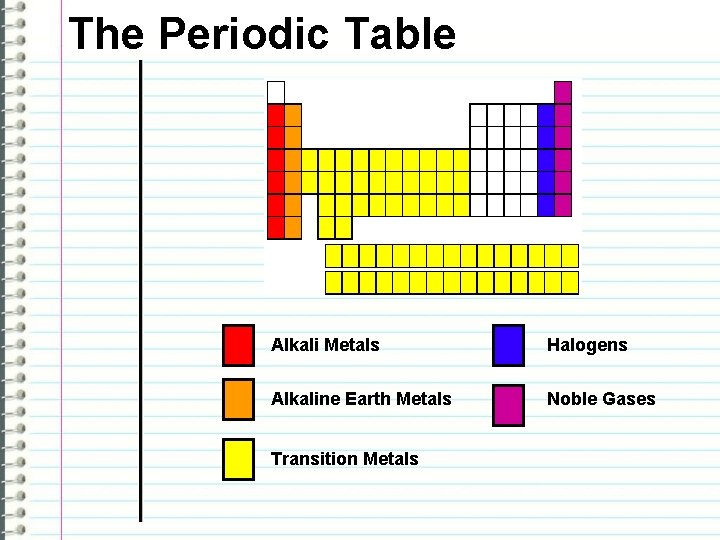
The Periodic Table Alkali Metals Halogens Alkaline Earth Metals Noble Gases Transition Metals
The Periodic Table States of Matter at Room Temperature: - Metals generally tend to be solid at room temperature, with the only exception being mercury - Semi-metals tend to be solid at room temperature as well - Non-metals in the upper right most corner tend to be gases (noble gases are obviously gases) - Bromine tends to be a liquid, hydrogen tends to be a gas

The Periodic Table Reactivity: - Groups 1 and 2 (alkali and alkali earth metals) are typically very reactive, especially in water -Halogens in the upper right corner also tend to be very reactive
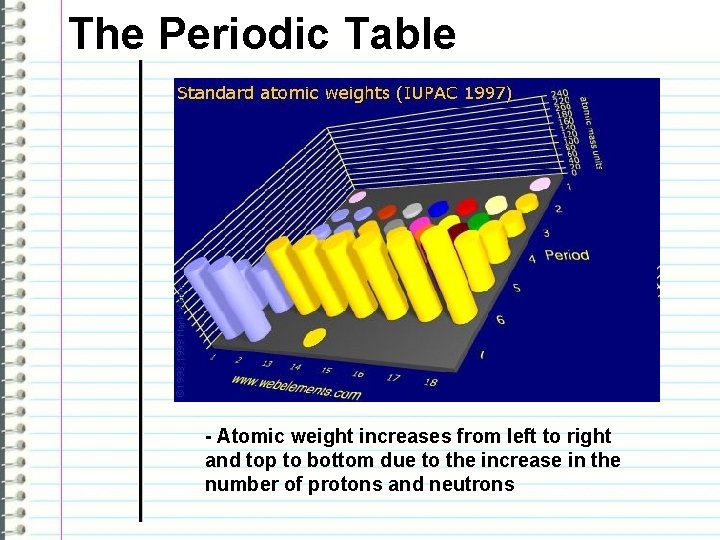
The Periodic Table - Atomic weight increases from left to right and top to bottom due to the increase in the number of protons and neutrons
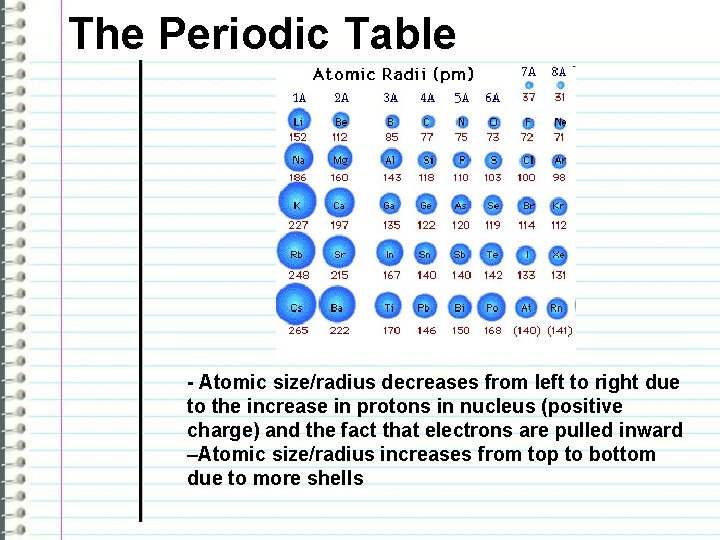
The Periodic Table - Atomic size/radius decreases from left to right due to the increase in protons in nucleus (positive charge) and the fact that electrons are pulled inward –Atomic size/radius increases from top to bottom due to more shells
- Slides: 10