The Periodic Table Early Periodic Table Simplest arrangement
The Periodic Table
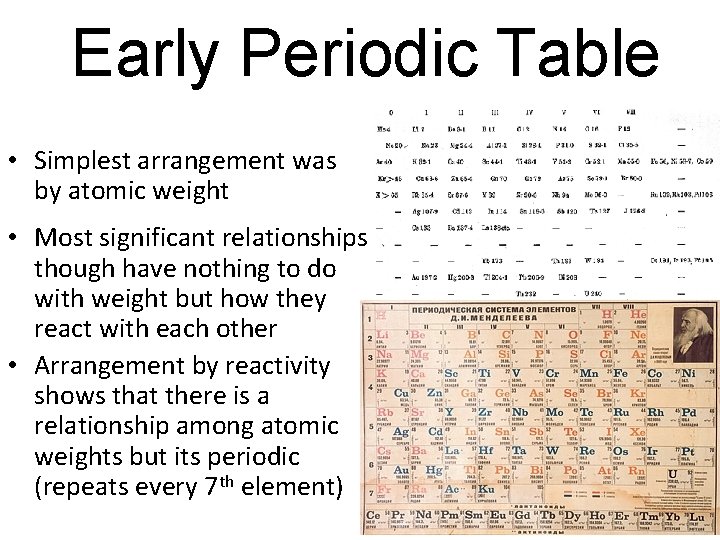
Early Periodic Table • Simplest arrangement was by atomic weight • Most significant relationships though have nothing to do with weight but how they react with each other • Arrangement by reactivity shows that there is a relationship among atomic weights but its periodic (repeats every 7 th element)
Periodic Law When elements are arranged by atomic number (# of p+) there is a repetition of chemical and physical properties
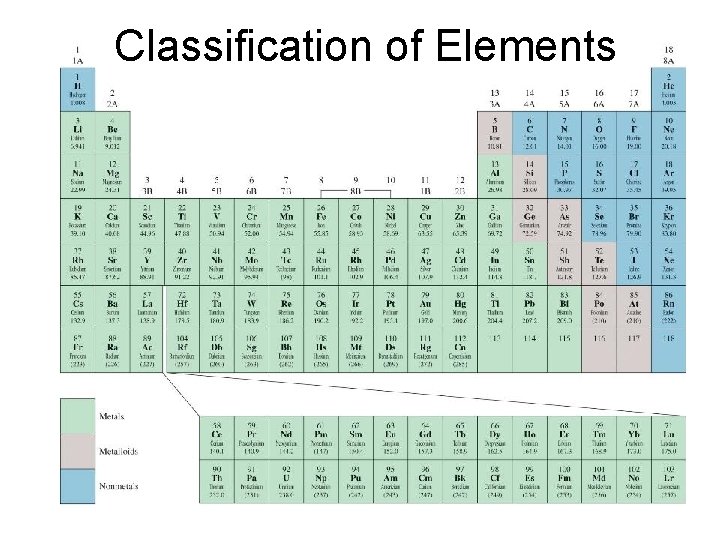
Classification of Elements

Metals • Properties – shiny, smooth, solids (except mercury) – Good conductors of heat and electricity – High densities, melting/boiling points – Malleable – bendt or pounded into sheets – Ductile – drawn into wires
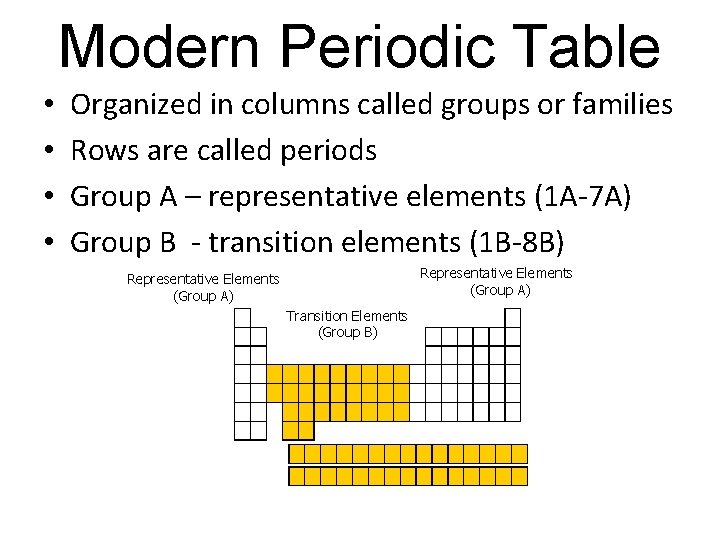
Modern Periodic Table • • Organized in columns called groups or families Rows are called periods Group A – representative elements (1 A-7 A) Group B - transition elements (1 B-8 B) Representative Elements (Group A) Transition Elements (Group B)
Nonmetals • Properties – Gases or brittle, dull looking solids – Poor conductors of heat and electricity – Usually have lower densities, melting point, and boiling point than metals sulfur
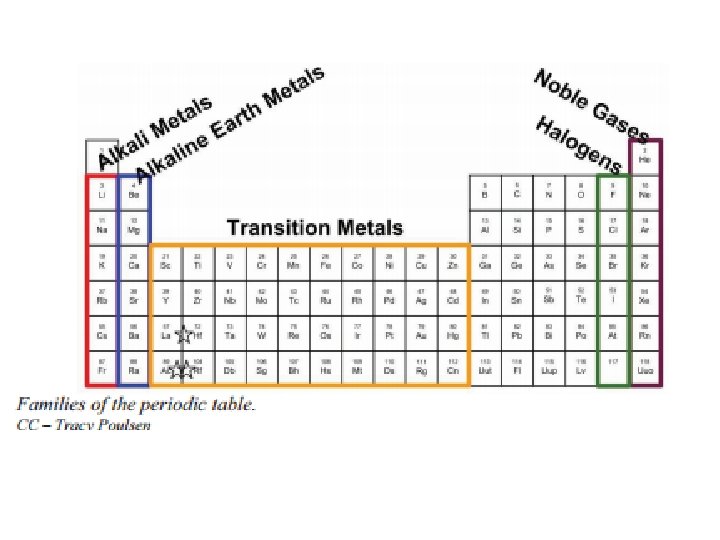

Metalloids (Semimetals) • Physical and chemical properties similar to both metals and nonmetals – They are metallic-looking brittle solids – Relatively good electrical conductivity.
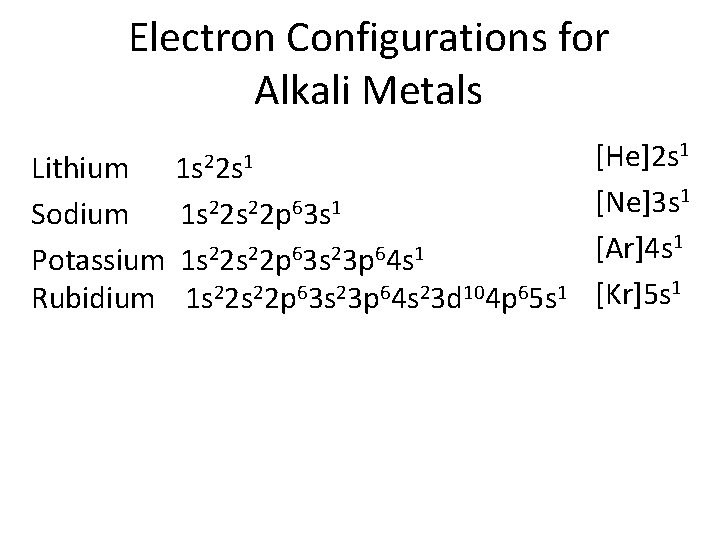
Electron Configurations for Alkali Metals 1 s 22 s 1 Lithium Sodium 1 s 22 p 63 s 1 Potassium 1 s 22 p 63 s 23 p 64 s 1 Rubidium 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 1 [He]2 s 1 [Ne]3 s 1 [Ar]4 s 1 [Kr]5 s 1

Electron Configurations for Alkaline Earth Metals 1 s 22 s 2 Beryllium Magnesium 1 s 22 p 63 s 2 Calcium 1 s 22 p 63 s 23 p 64 s 2 Strontium 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 2 [He]2 s 2 [Ne]3 s 2 [Ar]4 s 2 [Kr]5 s 2
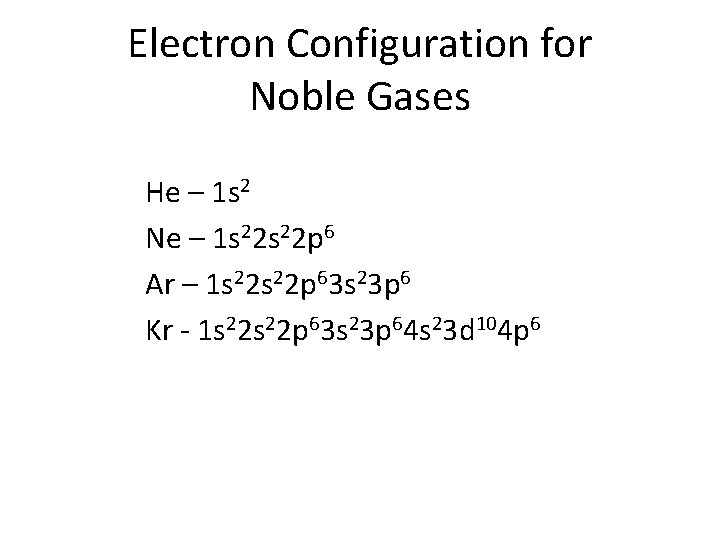
Electron Configuration for Noble Gases He – 1 s 2 Ne – 1 s 22 p 6 Ar – 1 s 22 p 63 s 23 p 6 Kr - 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6
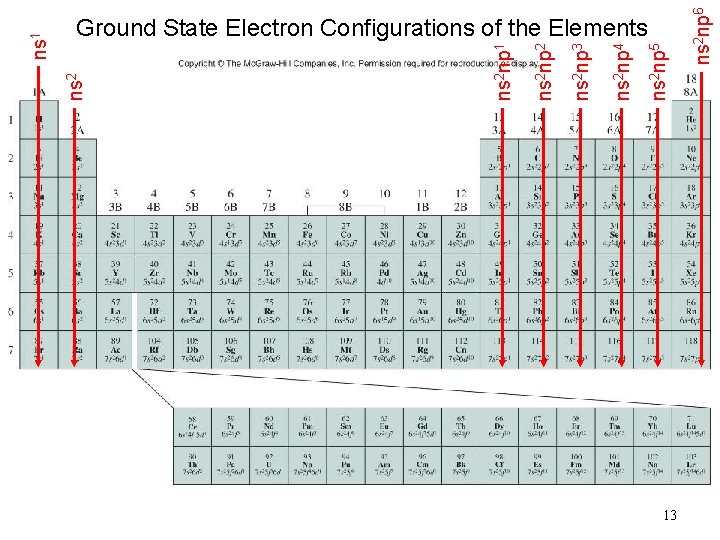
13 ns 2 np 6 ns 2 np 5 ns 2 np 4 ns 2 np 3 ns 2 np 2 ns 2 np 1 ns 2 ns 1 Ground State Electron Configurations of the Elements
Valence Electrons • Electrons in the outermost s and p orbitals (highest n shell) • These electrons participate in chemical reactions
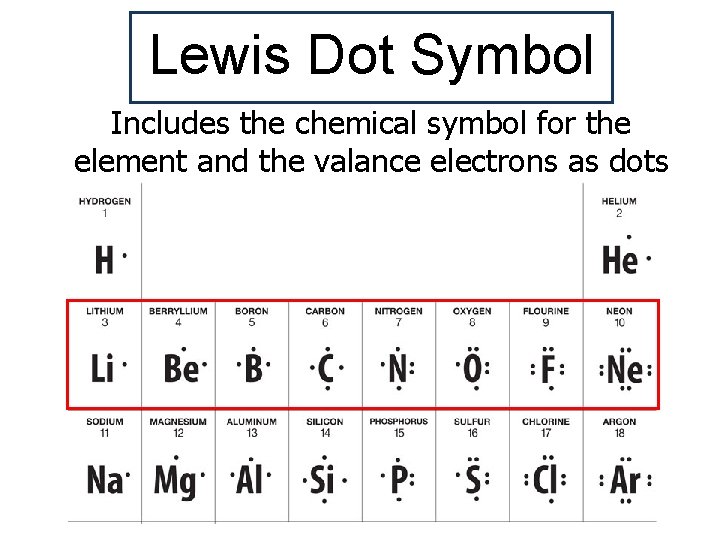
Lewis Dot Symbol Includes the chemical symbol for the element and the valance electrons as dots
Reactivity of Elements Atoms in the same group have similar chemical properties because they have the same number of valence electrons
Octet Rule Atoms gain, lose, or share electrons to acquire a full set of eight valence electrons (to be like a noble gas) Eight is great!!!
- Slides: 17