The Periodic Table Dmitri Mendeleev The first copy
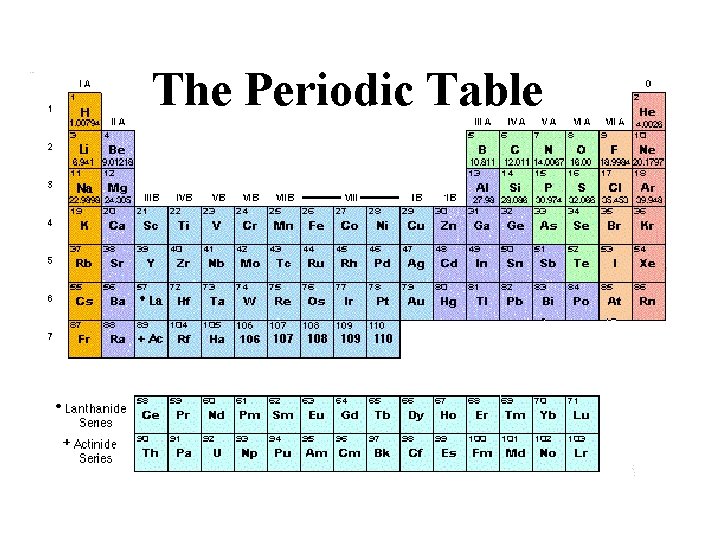
The Periodic Table

Dmitri Mendeleev • The first copy of the periodic table was published in 1869. • Mendeleev placed elements in order of increasing atomic mass. • Elements with similar properties fell into groups. • Mendeleev left gaps for elements that had not yet been discovered – gallium, scandium, and germanium
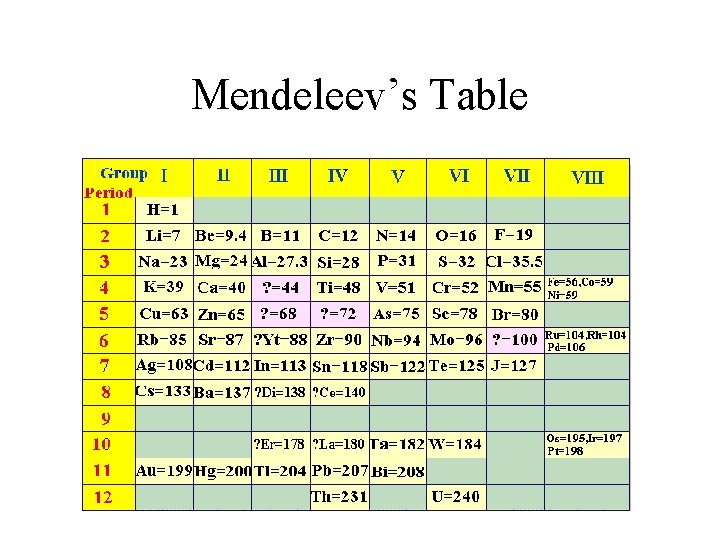
Mendeleev’s Table

Revising the Table • Henry Moseley rearranged the periodic table in order of increasing atomic number. • It was now more obvious how many elements had not yet been discovered.
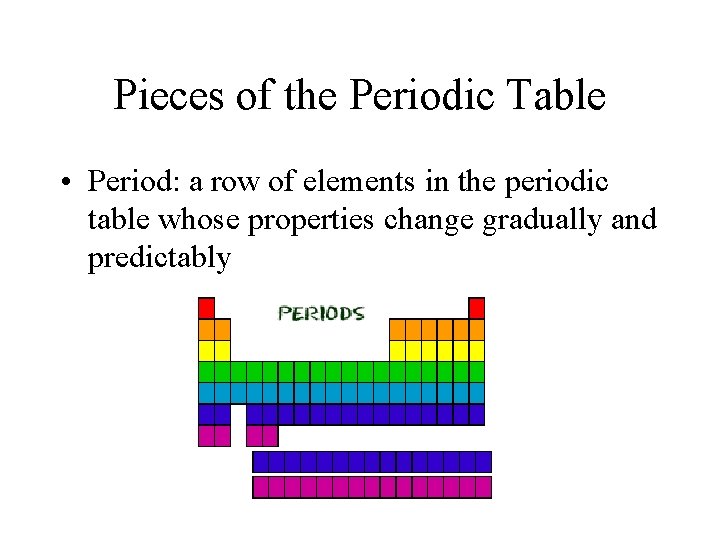
Pieces of the Periodic Table • Period: a row of elements in the periodic table whose properties change gradually and predictably
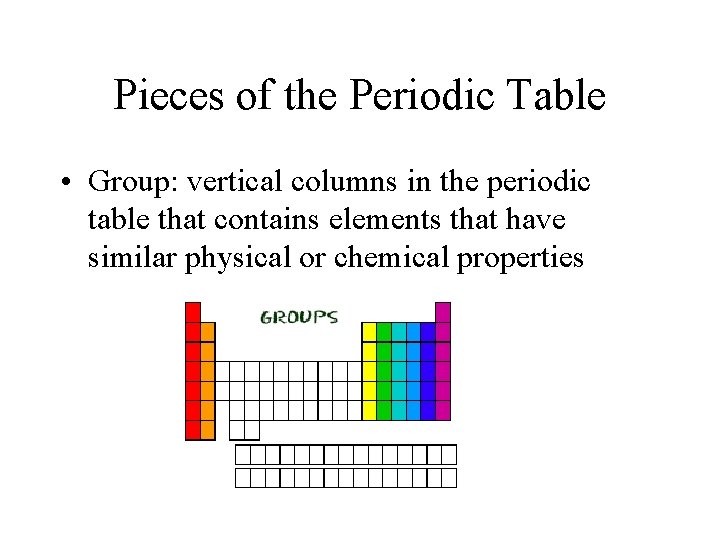
Pieces of the Periodic Table • Group: vertical columns in the periodic table that contains elements that have similar physical or chemical properties
Pieces of the Periodic Table • Representative elements: elements in Group 1 and 2 and Groups 13 thru 18 – These include metals, metalloids, and nonmetals • Transition Metals: The elements in Groups 3 thru 12 – These are all metals
Pieces of the Periodic Table • Inner Transition Metals: The group of elements placed below the periodic table – Lanthanide Series: top row – Actinide Series: bottom row

Latin Names of Symbols Iron Sodium Potassium Tin Silver Antimony Fe Na K Sn Ag Sb Ferrum Natrium Kalium Stannum Argentum Stibium

Latin Names of Symbols Tungsten Gold Mercury Lead Copper W Au Hg Pb Cu Wolfram Aurum Hydrargyrum Plumbum Cuprum
- Slides: 10