The Periodic Table Dmitri Mendeleev 1834 1907 Dmitri
The Periodic Table Dmitri Mendeleev (1834 1907)

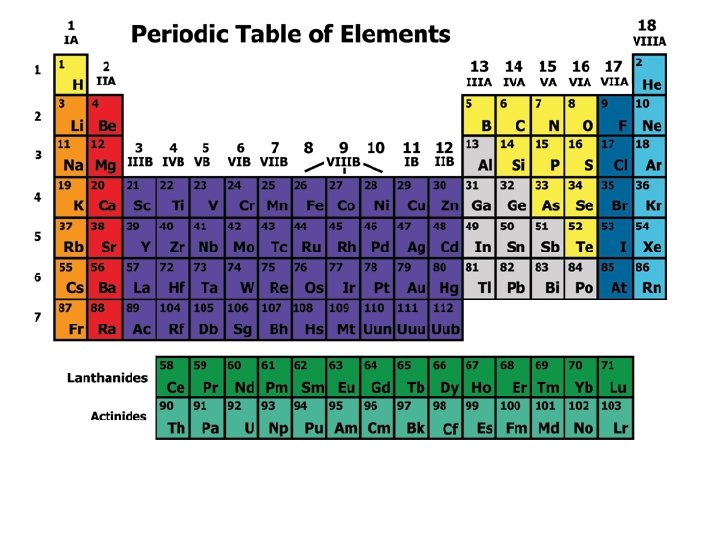
Dmitri Mendeleev • Mendeleev: A Russian Chemist. • 1869 published the 1 st version of the Periodic Table. • His Table showed that the elements are arranged in order of increasing atomic mass. • In order to make his table work, he had to leave 3 gaps for missing elements. • These gaps spurred chemists to look for the missing elements.

Henry Moseley • Early twentieth century, English physicist Moseley improved the table. • He arranged the elements according to the atomic number rather than atomic mass. • With Moseley’s table it was clear how many elements were missing.
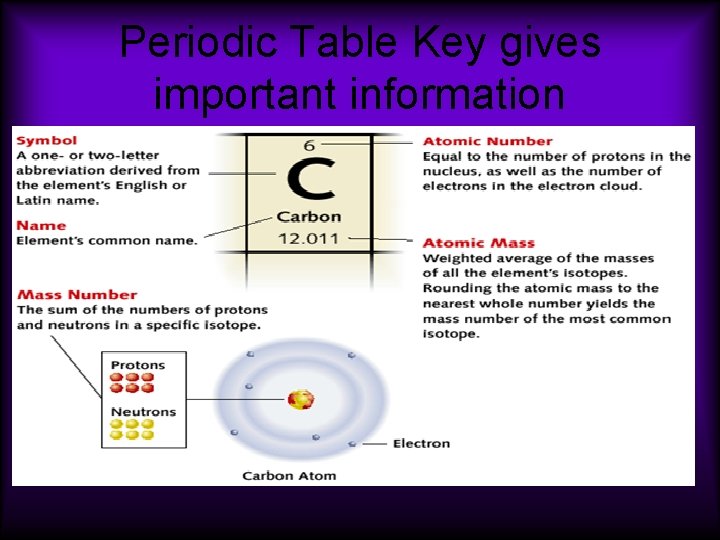
Periodic Table Key gives important information
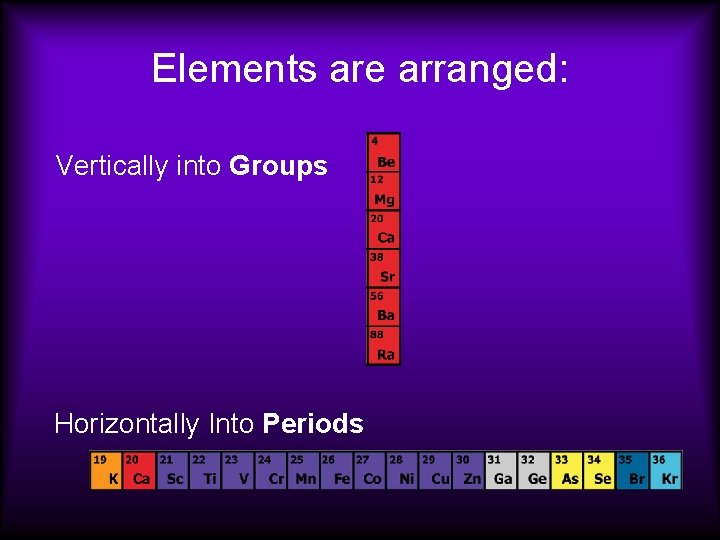
Elements are arranged: Vertically into Groups Horizontally Into Periods

Why?
If you looked at one atom of every element in a group you would see…

Each atom has the same number of electrons in its outermost shell. • An example…
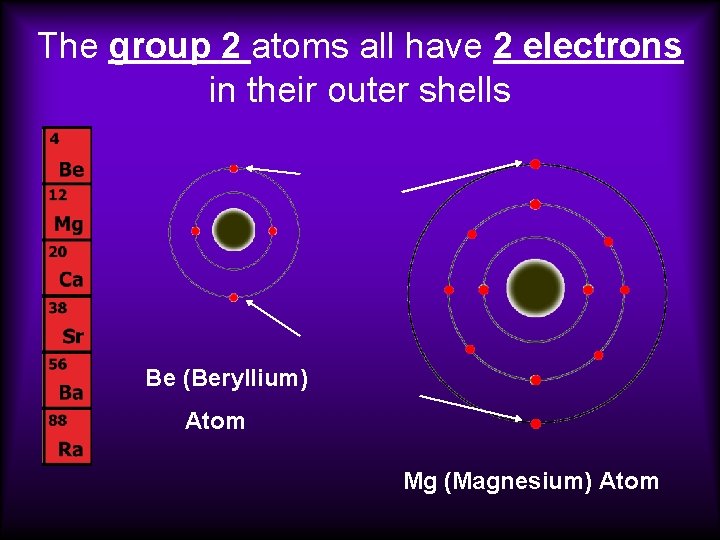
The group 2 atoms all have 2 electrons in their outer shells Be (Beryllium) Atom Mg (Magnesium) Atom

• The number of outer or “valence” electrons in an atom affects the way an atom bonds. • The way an atom bonds determines many properties of the element. • This is why elements within a group usually have similar properties.
If you looked at an atom from each element in a period you would see…
Each atom has the same number of electron shells. An example…
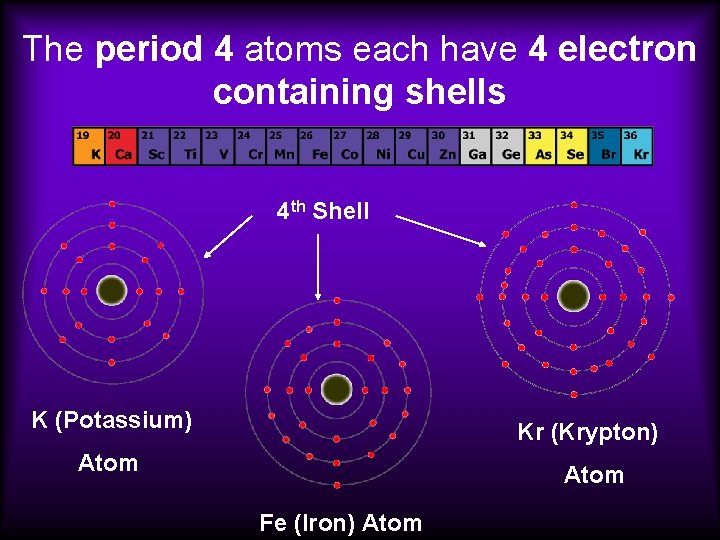
The period 4 atoms each have 4 electron containing shells 4 th Shell K (Potassium) Kr (Krypton) Atom Fe (Iron) Atom

Each group has distinct properties • The periodic Table is divided into several groups based on the properties of different atoms.
Alkali Metals Soft, silvery colored metals Very reactive!!!

Group 1 A: Alkali Metals Reaction of potassium + H 2 O Cutting sodium metal
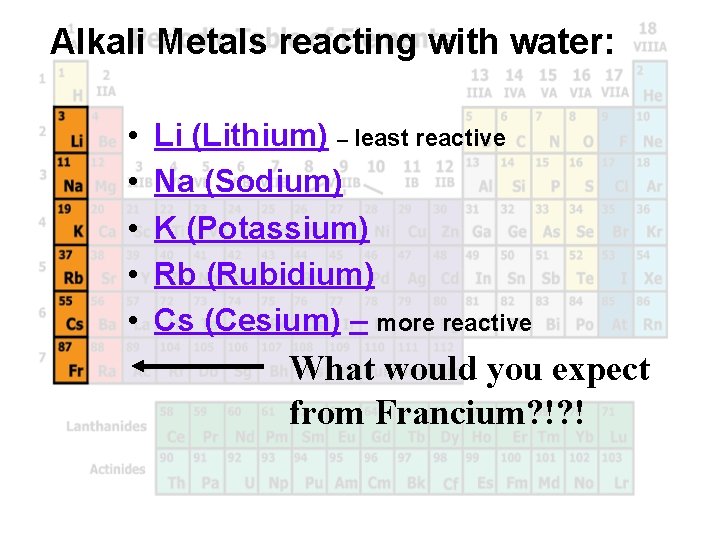
Alkali Metals reacting with water: • • • Li (Lithium) – least reactive Na (Sodium) K (Potassium) Rb (Rubidium) Cs (Cesium) – more reactive What would you expect from Francium? !? !
Group 2 A: Alkaline Earth Metals Magnesium oxide
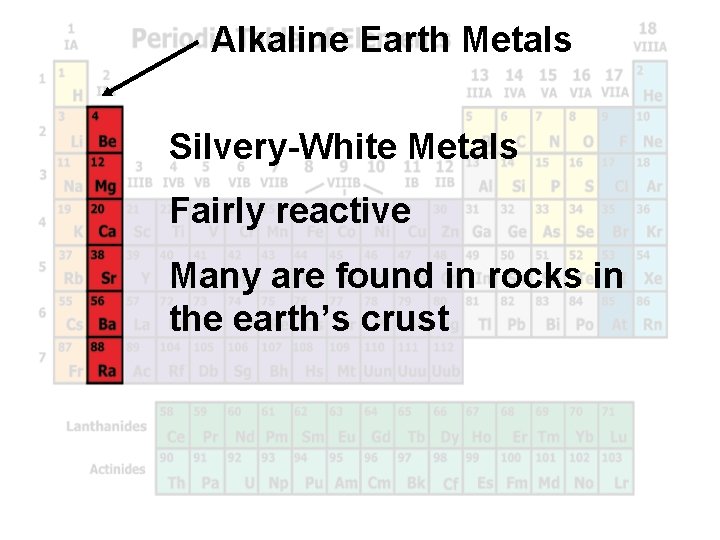
Alkaline Earth Metals Silvery-White Metals Fairly reactive Many are found in rocks in the earth’s crust
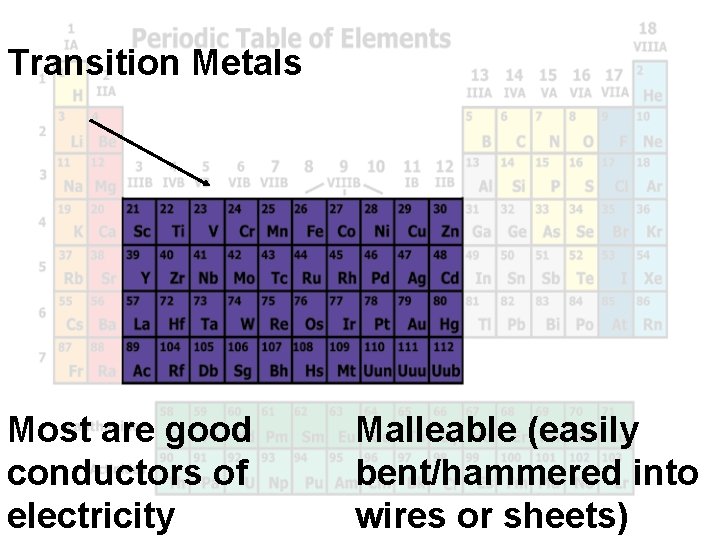
Transition Metals Most are good conductors of electricity Malleable (easily bent/hammered into wires or sheets)

How many things can you think of that have Transition Metals in them?

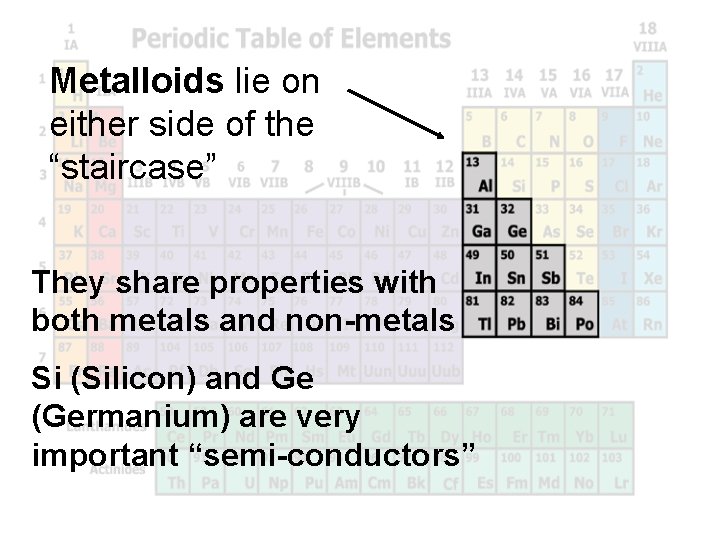
Metalloids lie on either side of the “staircase” They share properties with both metals and non-metals Si (Silicon) and Ge (Germanium) are very important “semi-conductors”

What are semiconductors used in?
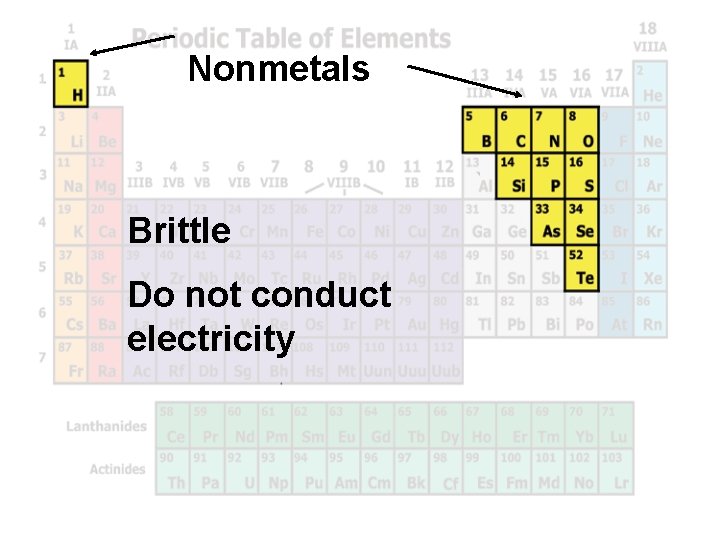
Nonmetals Brittle Do not conduct electricity
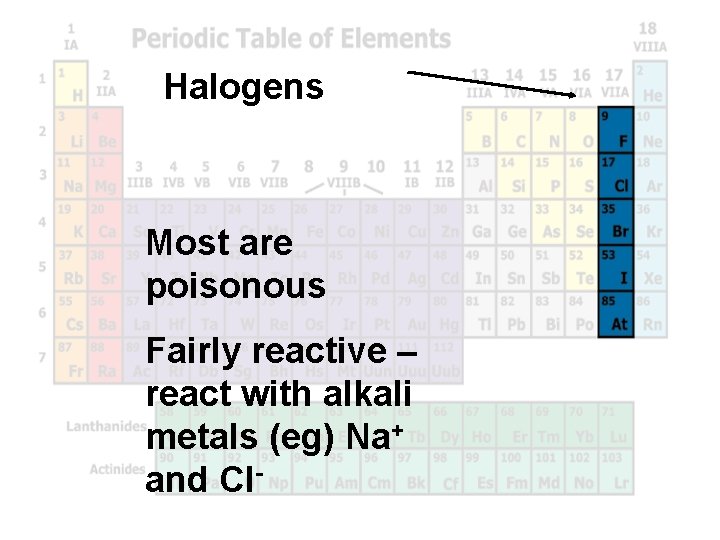
Halogens Most are poisonous Fairly reactive – react with alkali metals (eg) Na+ and Cl-
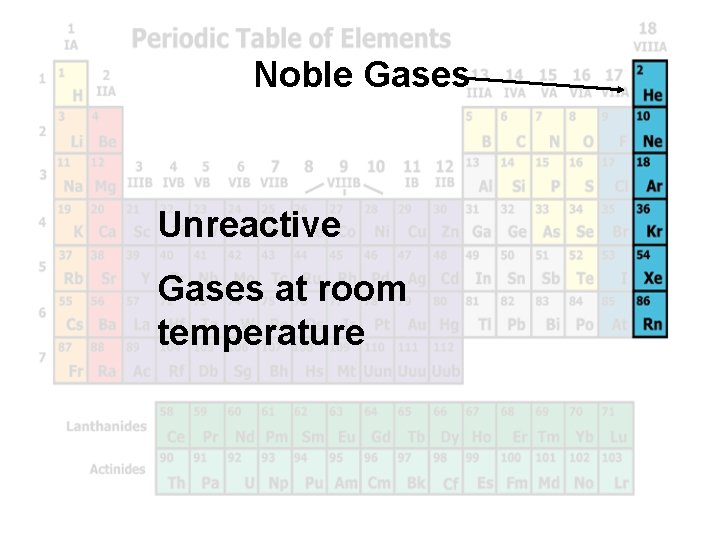
Noble Gases Unreactive Gases at room temperature

Jellyfish lamps made with noble gases artist- Eric Ehlenberger

Colors Noble Gases produce in lamp tubes: • Ne (Neon): orange-red • Hg (Mercury): light blue • Ar (Argon): pale lavender • He (Helium): pale peach • Kr (Krypton): pale silver • Xe (Xenon): pale, deep blue
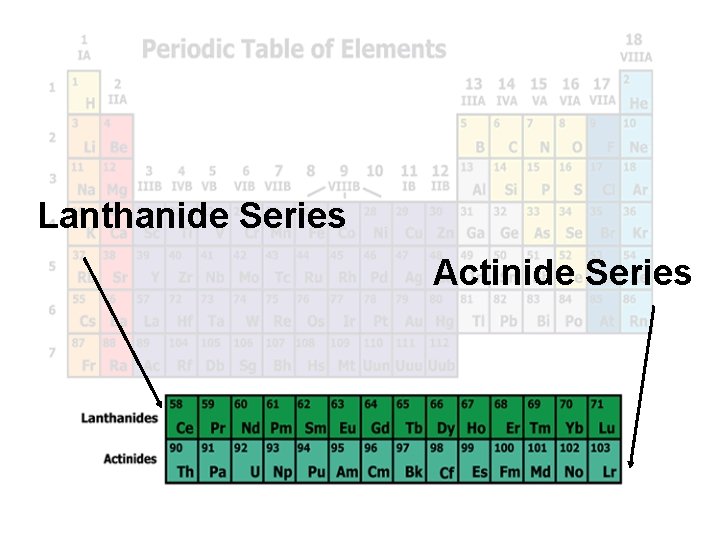
Lanthanide Series Actinide Series
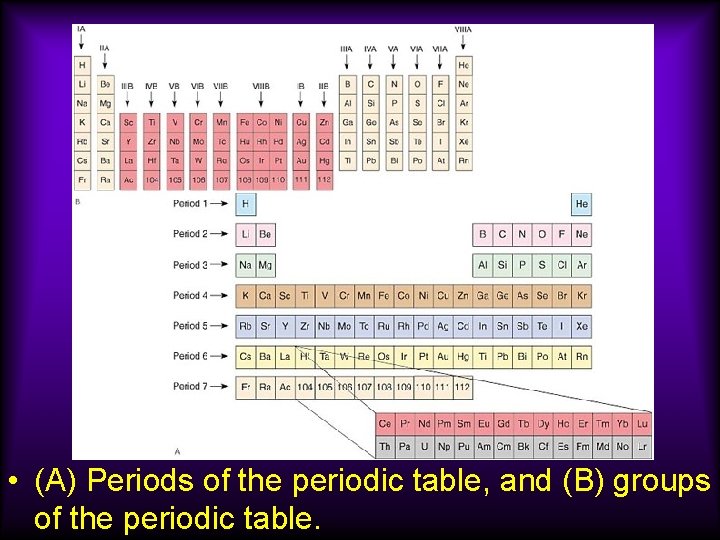
• (A) Periods of the periodic table, and (B) groups of the periodic table.

Metal: Elements that are usually solids at room temperature. Most elements are metals. Non-Metal: Elements in the upper right corner of the periodic Table. Their chemical and physical properties are different from metals. Metalloid: Elements that lie on a diagonal line between the metals and non-metals. Their chemical and physical properties are intermediate between the two.

Compounds – composed of 2 or more elements in a fixed ratio – properties differ from those of individual elements – EX: table salt (Na. Cl)
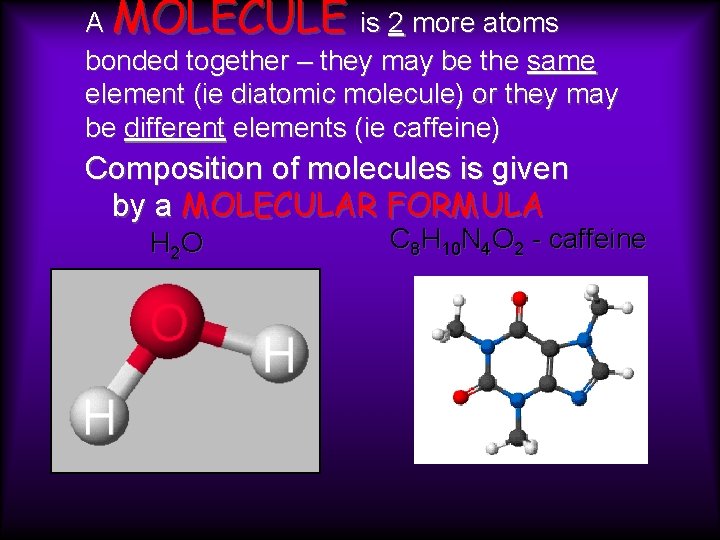
A MOLECULE is 2 more atoms bonded together – they may be the same element (ie diatomic molecule) or they may be different elements (ie caffeine) Composition of molecules is given by a MOLECULAR FORMULA H 2 O C 8 H 10 N 4 O 2 - caffeine
- Slides: 35