The Periodic Table Developing the Periodic Table The

The Periodic Table
Developing the Periodic Table • The arrangement of the elements in the periodic table is now understood in terms of the underlying atomic structure of the elements, but the table was initially developed from experimental observations of the physical and chemical properties of the various elements.

Developing the Periodic Table • In 1869, Dmitri Mendeleev observed that, when the elements are arranged in order of increasing atomic weight, elements with similar properties appear in a regular pattern. He noticed a periodicity (periodic repetition) in the properties of the elements. • Dmitri Mendeleev

Developing the Periodic Table • In Mendeleev’s original table, he left empty spaces where it seemed to him an undiscovered element would fit. He made predictions about the properties for these missing elements. For example, he left a space between Silicon and Tin in Group 4 A for an element he called ekasilicon. Germanium was discovered in 1886 and confirmed the predictions he made.
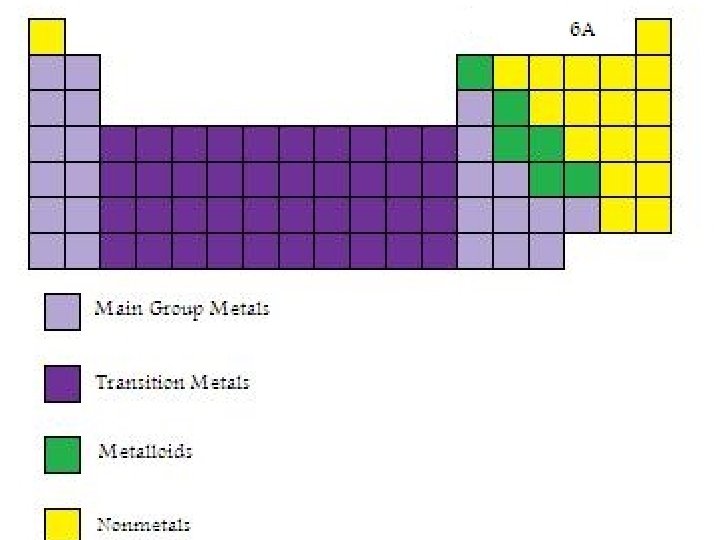
Features of the Periodic Table • Elements are arranged so those with similar chemical and physical properties lie in vertical columns called groups or families. The periodic table commonly used in the United States has groups numbered 1 through 8, with each number followed by a letter: A or B. The A groups are often called the main group elements and the B groups are the transition elements. • The horizontal rows of the table are called periods, and they are numbered beginning with 1 for the period containing H and He.
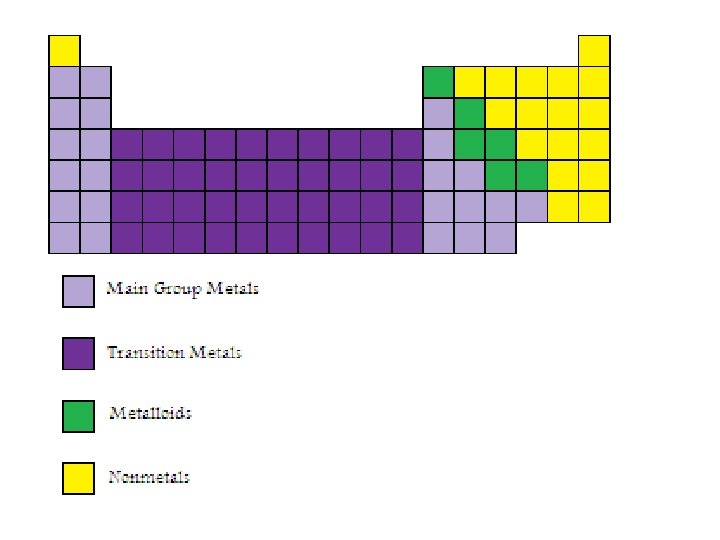

Metals, Non-metals and Metalloids • Metals are solids at room temperature (except for mercury), can conduct electricity, are usually ductile (can be drawn into wires) and malleable (can be formed into sheets) and can form alloys (solutions of more than one metal). • Non-metals are mostly solids are room temperature. Five of them are gases at room temperature and one is a liquid. With the exception of carbon in the form of graphite, nonmetals do not conduct electricity, which is one of the main features that distinguishes metals from non-metals. • Metalloids have properties that make it difficult to distinguish whether they are metals or non-metals.
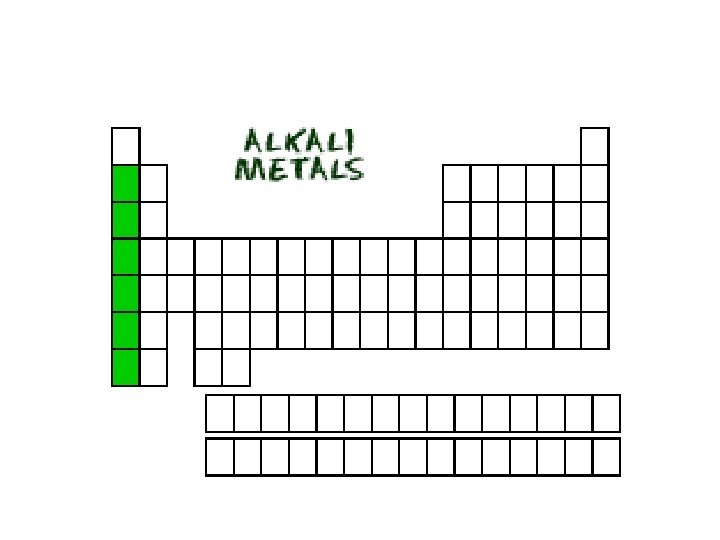

Group 1 A Elements • The metals in the leftmost column, Group 1 A, are known as the alkali metals. Each is a metal and each is a solid at room temperature. Each is very reactive and as a result is only found in nature as part of a compound and never as a free element.

Group 2 A Elements • The elements of the second group, Group 2 A, are known as alkaline earth metals. Similarly to the alkali metals, they are only found in compounds in nature.
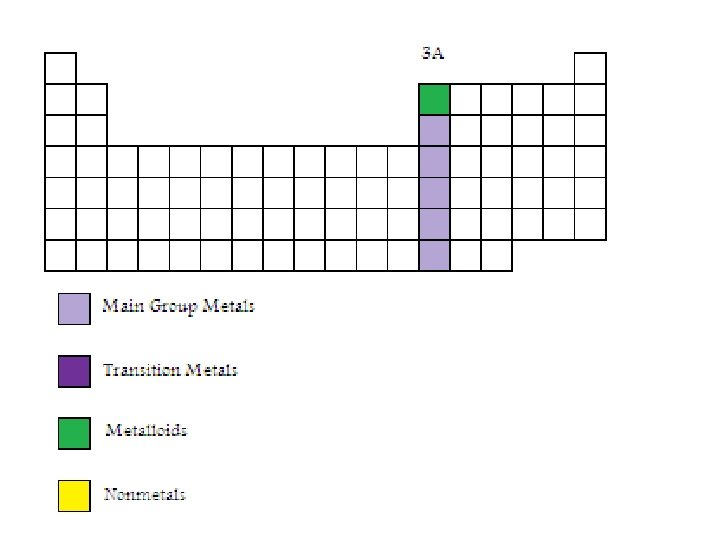

Group 3 A Elements • Boron, aluminum, gallium, indium and thallium are elements in Group 3 A. Aluminum is the most abundant metal in the earth’s crust. Boron is a metalloid while the others are metals.
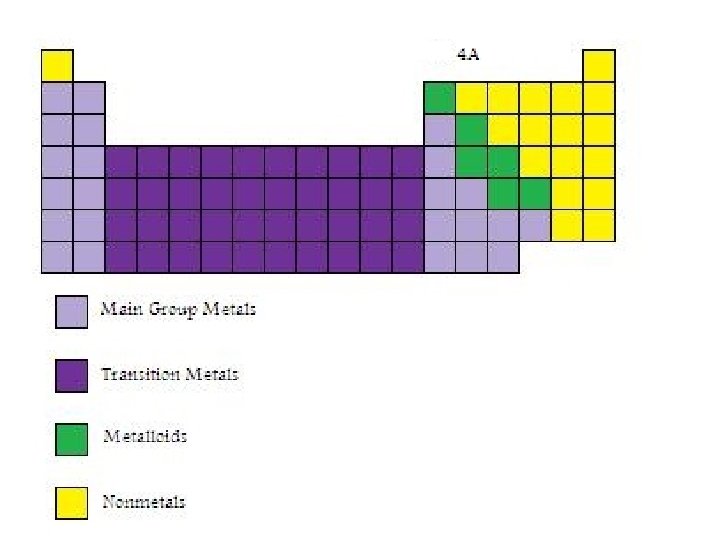

Group 4 A Elements • Group 4 A contains a non-metal, two metalloids and several metals.
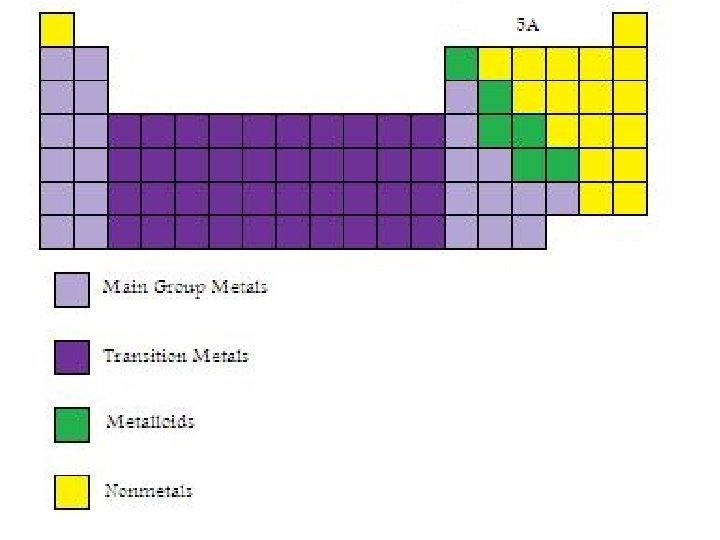

Group 5 A Elements

Group 6 A Elements • The Group 6 A elements are also known as chalcogens.
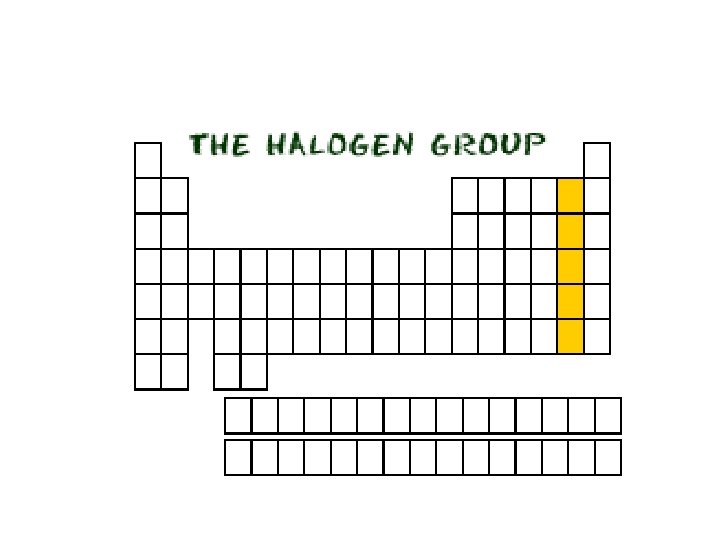

Group 7 A Elements • Group 7 A is composed entirely of non-metals. Similarly to Group 1 A these elements are very reactive. The Group 7 A elements are also known as halogens. The word comes from the Greek meaning “salt” “forming”.
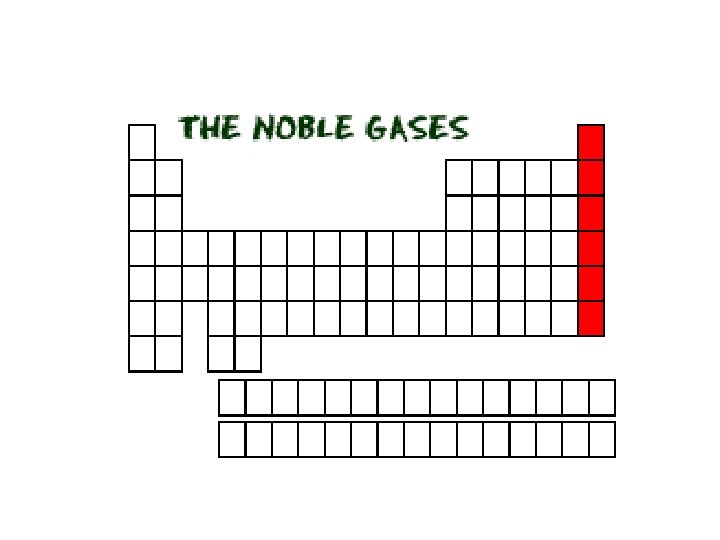

Group 8 A Elements • The Group 8 A elements are also known as the noble gases. They were given this name because of their general lack of reactivity.
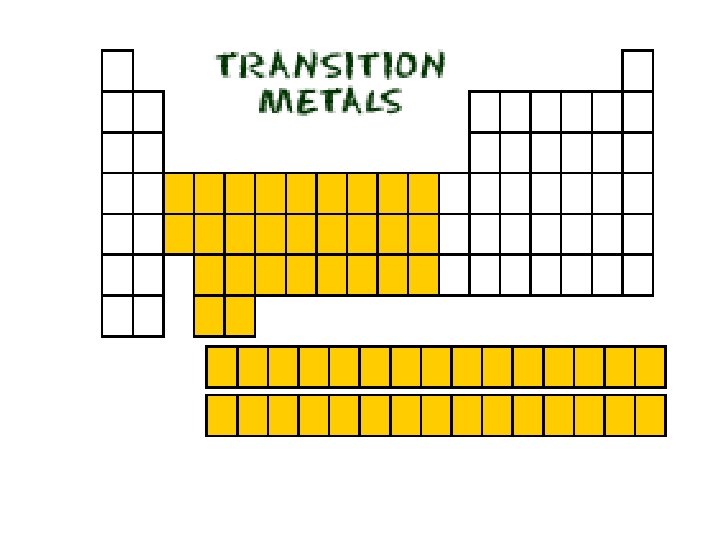
Groups 1 B through 8 B elements • All of these elements are metals.
- Slides: 25