The periodic table Describe the chemical reactions that
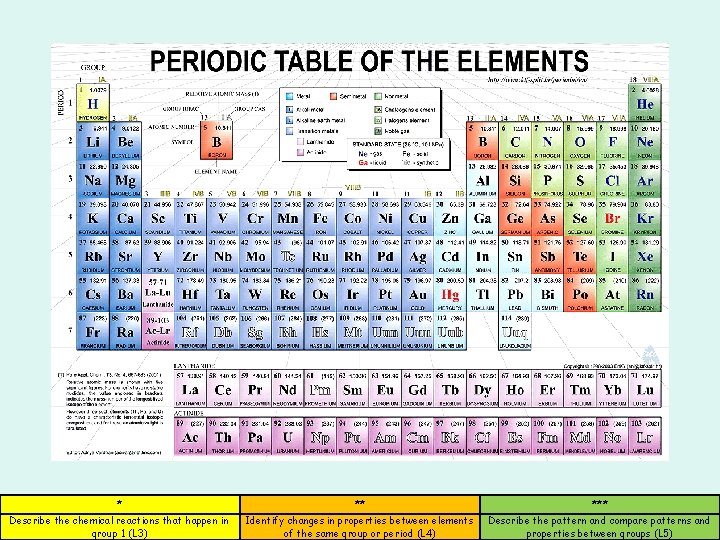
The periodic table * ** *** Describe the chemical reactions that happen in group 1 (L 3) Identify changes in properties between elements of the same group or period (L 4) Describe the pattern and compare patterns and properties between groups (L 5)
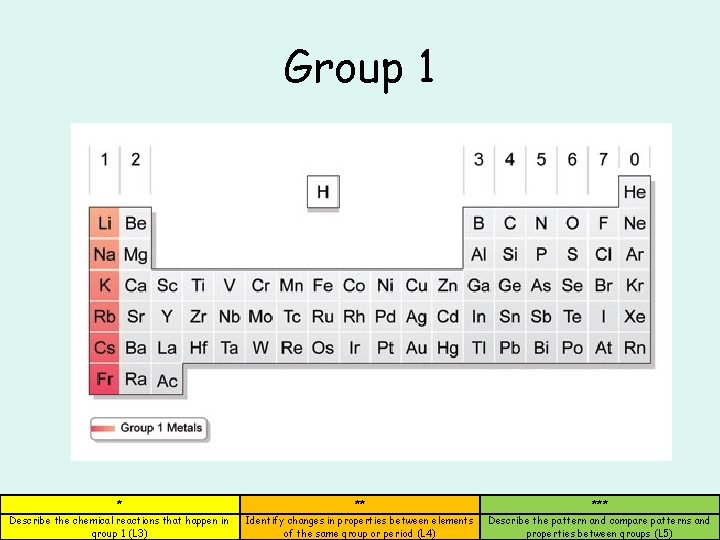
Group 1 * ** *** Describe the chemical reactions that happen in group 1 (L 3) Identify changes in properties between elements of the same group or period (L 4) Describe the pattern and compare patterns and properties between groups (L 5)
Trends in group 1 metals • What trends do you see as you go down the group? • What do the metals all have in common? • What other properties would you expect the elements in group 1 to have as they are all metals? * ** *** Describe the chemical reactions that happen in group 1 (L 3) Identify changes in properties between elements of the same group or period (L 4) Describe the pattern and compare patterns and properties between groups (L 5)
* ** *** Describe the chemical reactions that happen in group 1 (L 3) Identify changes in properties between elements of the same group or period (L 4) Describe the pattern and compare patterns and properties between groups (L 5)
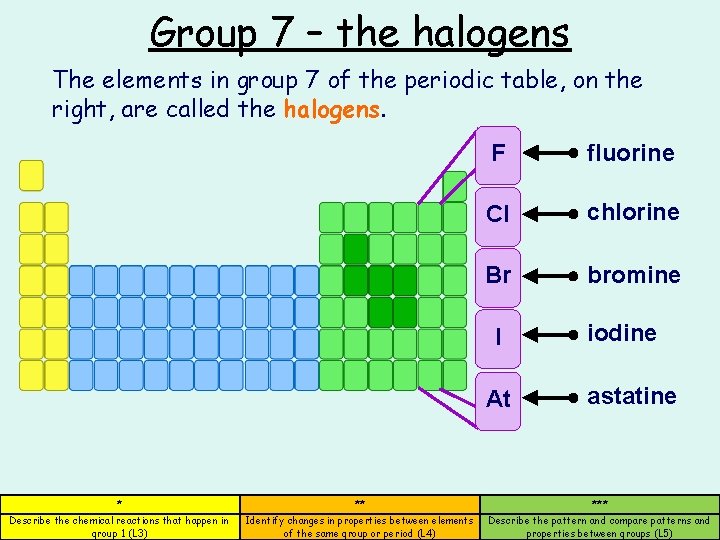
Group 7 – the halogens The elements in group 7 of the periodic table, on the right, are called the halogens. F fluorine Cl chlorine Br bromine I At iodine astatine * ** *** Describe the chemical reactions that happen in group 1 (L 3) Identify changes in properties between elements of the same group or period (L 4) Describe the pattern and compare patterns and properties between groups (L 5)
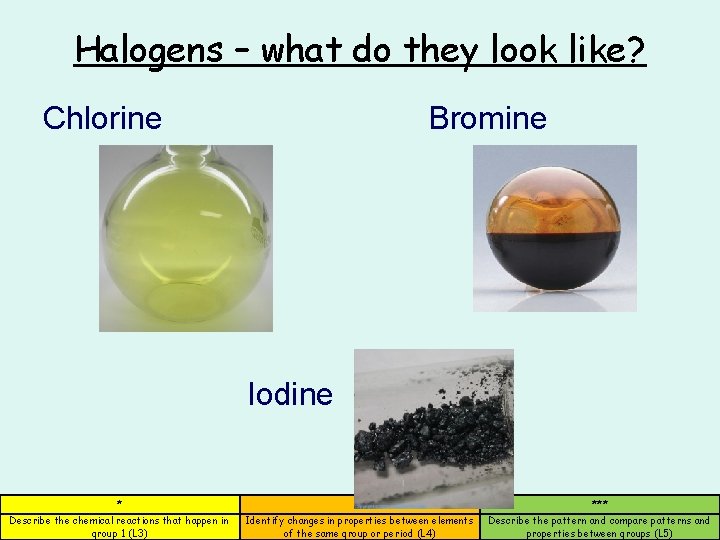
Halogens – what do they look like? Chlorine Bromine Iodine * ** *** Describe the chemical reactions that happen in group 1 (L 3) Identify changes in properties between elements of the same group or period (L 4) Describe the pattern and compare patterns and properties between groups (L 5)
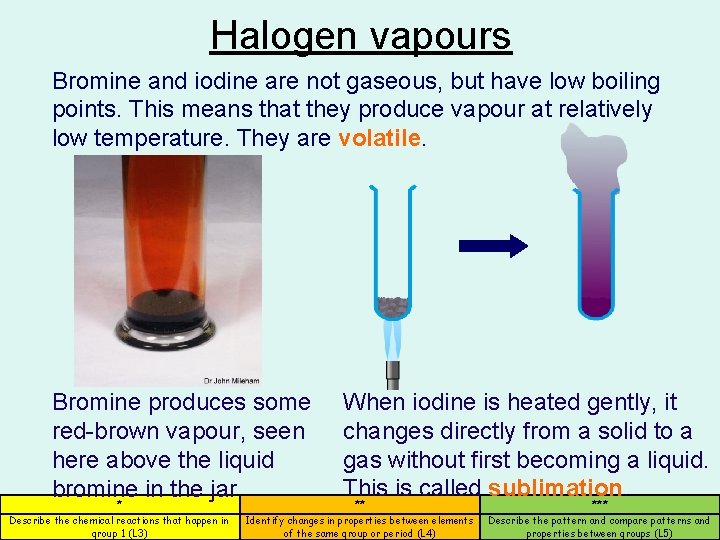
Halogen vapours Bromine and iodine are not gaseous, but have low boiling points. This means that they produce vapour at relatively low temperature. They are volatile. Bromine produces some red-brown vapour, seen here above the liquid bromine in the jar. * Describe the chemical reactions that happen in group 1 (L 3) When iodine is heated gently, it changes directly from a solid to a gas without first becoming a liquid. This is called sublimation. ** *** Identify changes in properties between elements of the same group or period (L 4) Describe the pattern and compare patterns and properties between groups (L 5)
Iodine fingerprints * ** *** Describe the chemical reactions that happen in group 1 (L 3) Identify changes in properties between elements of the same group or period (L 4) Describe the pattern and compare patterns and properties between groups (L 5)
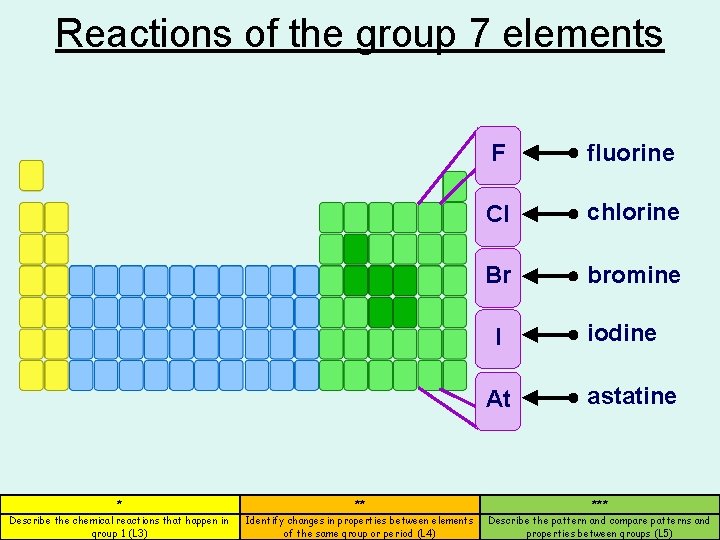
Reactions of the group 7 elements F fluorine Cl chlorine Br bromine I At iodine astatine * ** *** Describe the chemical reactions that happen in group 1 (L 3) Identify changes in properties between elements of the same group or period (L 4) Describe the pattern and compare patterns and properties between groups (L 5)

Watch the video • You will be asked to put the halogens in order of reactivity * ** *** Describe the chemical reactions that happen in group 1 (L 3) Identify changes in properties between elements of the same group or period (L 4) Describe the pattern and compare patterns and properties between groups (L 5)
Halogens • Chlorine • Iodine • Bromine • Fluorine * ** *** Describe the chemical reactions that happen in group 1 (L 3) Identify changes in properties between elements of the same group or period (L 4) Describe the pattern and compare patterns and properties between groups (L 5)
* ** *** Describe the chemical reactions that happen in group 1 (L 3) Identify changes in properties between elements of the same group or period (L 4) Describe the pattern and compare patterns and properties between groups (L 5)

Group 0 • This is also called group 8 • How are group 8 elements different to group 1 or 7? * ** *** Describe the chemical reactions that happen in group 1 (L 3) Identify changes in properties between elements of the same group or period (L 4) Describe the pattern and compare patterns and properties between groups (L 5)
- Slides: 13