The Periodic Table Chapter 6 www webelements com

The Periodic Table Chapter 6 www. webelements. com
Why is the Periodic Table important to me? n The periodic table is the most useful tool to a chemist. n You get to use it on every test. n It organizes lots of information about all the known elements.
Pre-Periodic Table Chemistry … n …was a mess!!! n No organization of elements. n Imagine going to a grocery store with no organization!! n Difficult to find information. n Chemistry didn’t make sense.
ORGANIZING THE ELEMENTS n J. W. Dobereiner (a German) in 1829 used the chemical properties of the elements to sort them in groups of threes (triads). n Dimitri Mendeleev (a Russian) in 1869 used increasing atomic mass to order the elements. n (Remember proton was not discovered until 1886—nucleus in 1911. )
Dmitri Mendeleev: Father of the Table HOW HIS WORKED… n Put elements in rows by increasing atomic mass. n Put elements in columns by the way they reacted. SOME PROBLEMS… n He left blank spaces for what he said were undiscovered elements. (Turned out he was right!) n He broke the pattern of increasing atomic mass to keep similar reacting elements together.
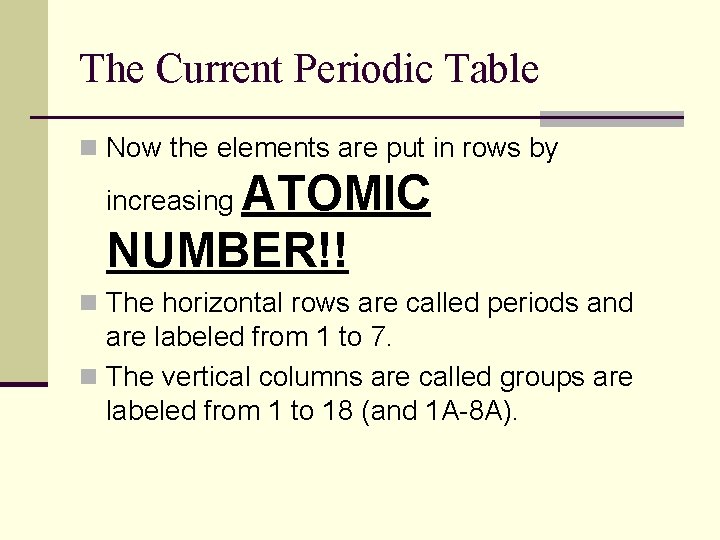
The Current Periodic Table n Now the elements are put in rows by ATOMIC NUMBER!! increasing n The horizontal rows are called periods and are labeled from 1 to 7. n The vertical columns are called groups are labeled from 1 to 18 (and 1 A-8 A).
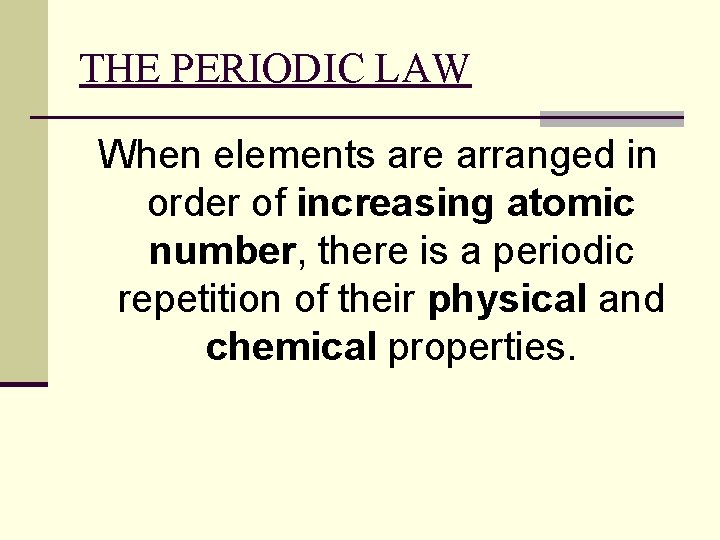
THE PERIODIC LAW When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties.
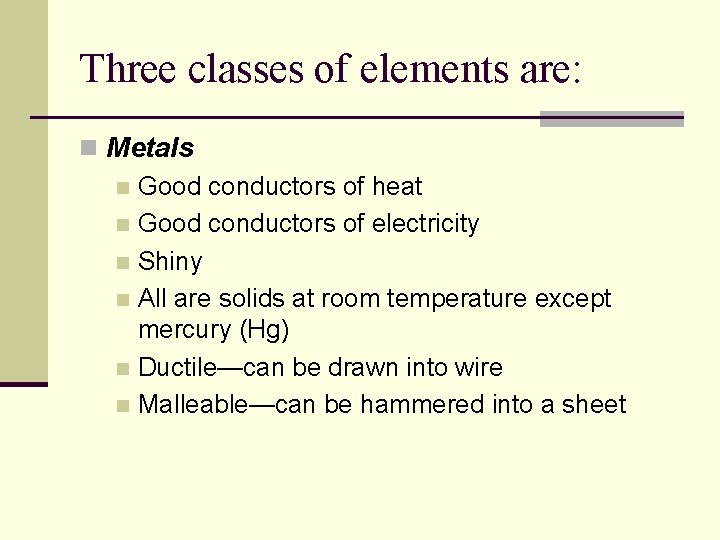
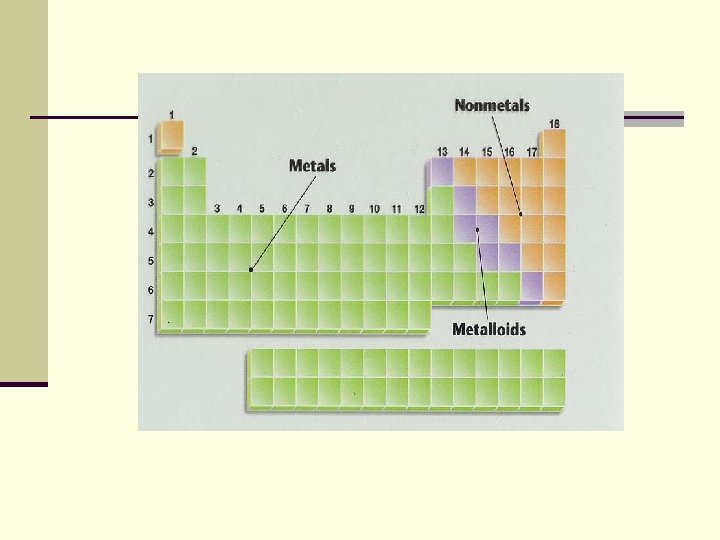
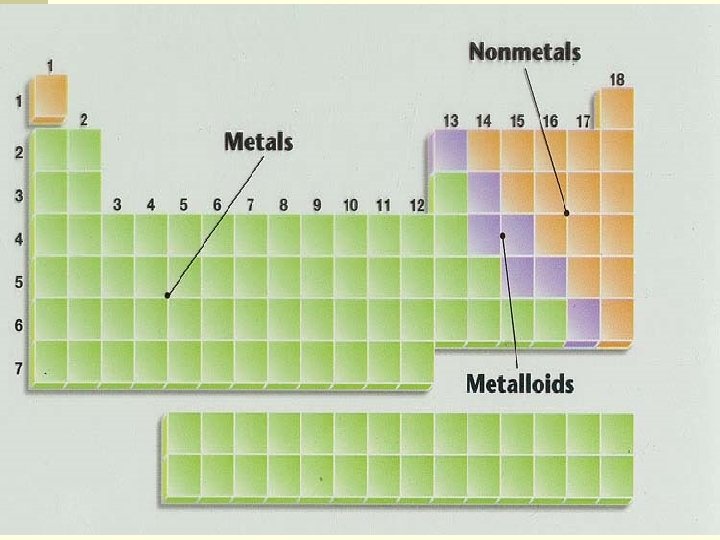
Three classes of elements are: n Metals n Good conductors of heat n Good conductors of electricity n Shiny n All are solids at room temperature except mercury (Hg) n Ductile—can be drawn into wire n Malleable—can be hammered into a sheet
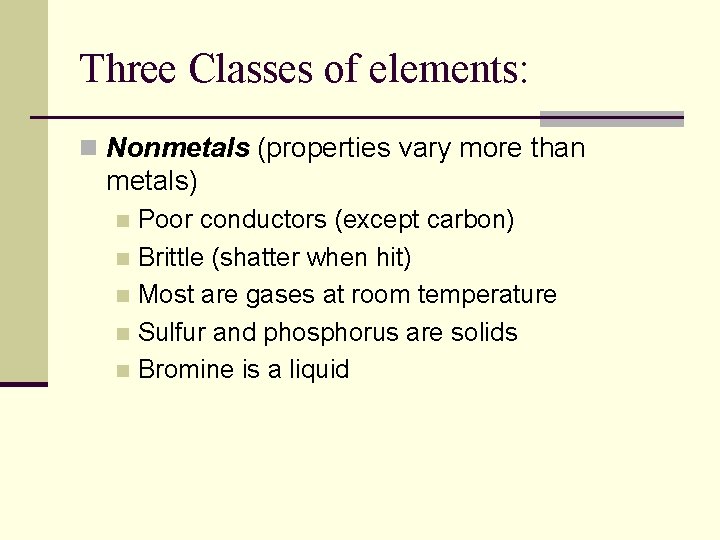
Three Classes of elements: n Nonmetals (properties vary more than metals) Poor conductors (except carbon) n Brittle (shatter when hit) n Most are gases at room temperature n Sulfur and phosphorus are solids n Bromine is a liquid n

Three Classes of elements n Metalloids n They behave as metals and nonmetals depending on conditions n If boron is added to silicon, then silicon will conduct electricity = semiconductor
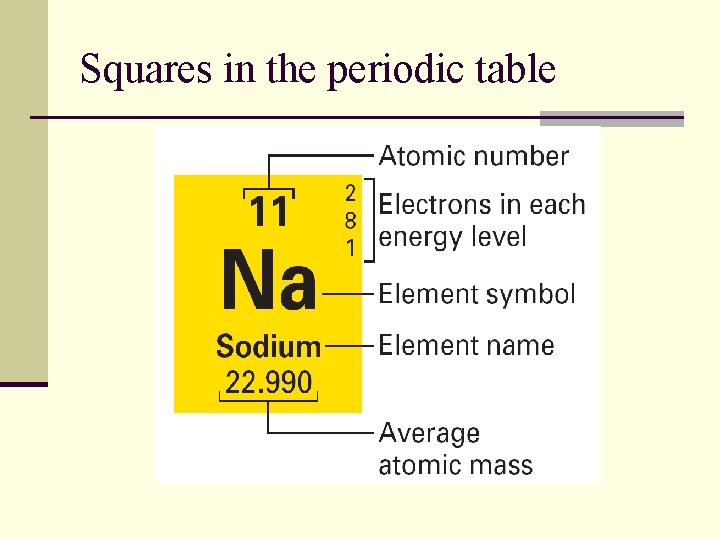
Squares in the periodic table
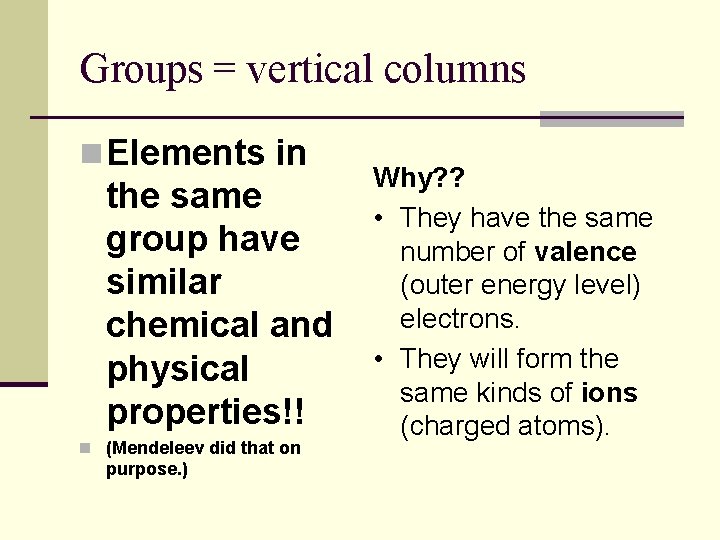
Groups = vertical columns n Elements in the same group have similar chemical and physical properties!! n (Mendeleev did that on purpose. ) Why? ? • They have the same number of valence (outer energy level) electrons. • They will form the same kinds of ions (charged atoms).

Reactivity GROUPS n REACTIVITY INCREASES AS YOU GO DOWN A GROUP OF METALS n REACTIVITY DECREASES AS YOU GO DOWN A GROUP OF NONMETALS
Alkali Metals –s 1 (2 s 1 – 7 s 1) n 1 st column on the periodic table (Group 1 or Group 1 A) not including hydrogen. n Very reactive metals, always combined with something else in nature (like in salt). n Soft enough to cut with a butter knife

Alkali metals

Cesium in water
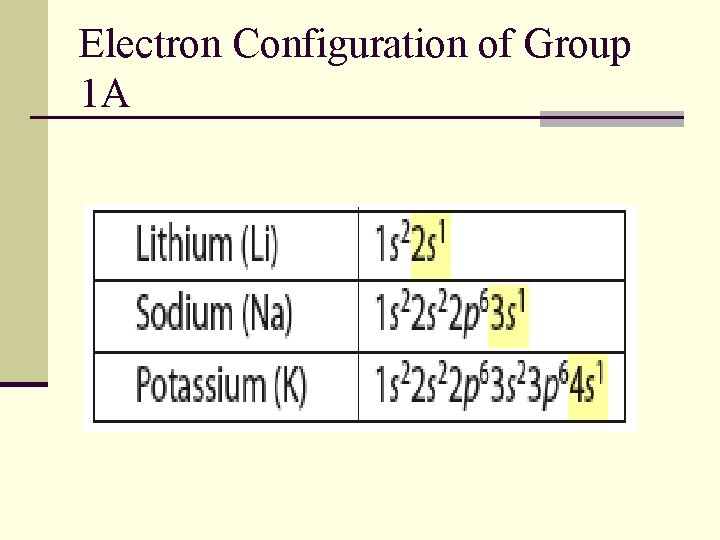
Electron Configuration of Group 1 A

Hydrogen n Hydrogen belongs to a family of its own. n Hydrogen is a diatomic —always H 2, reactive gas. n Hydrogen was involved in the explosion of the Hindenberg. n Hydrogen is promising as an alternative fuel source for automobiles
Alkaline Earth Metals –s 2 (2 s 2 - 3 s 2 ) n Second column on the periodic table. (Group 2 or 2 A) n Reactive metals that are always combined with nonmetals in nature. n Several of these elements are important mineral nutrients (such as Mg and Ca

Alkaline Earth Metals
Transition Metals (d-block d 1 - d 10) n Elements in groups 3 -12 n Less reactive harder metals n Includes metals used in jewelry and construction. n Metals used as “uncombined” element metal. n The transition elements often act as catalysts in reactions and are often colorful in compounds.
Transition metals n The Group B elements
Boron Family p 1 (2 p 1 - 6 p 1 ) Elements in group 13 (3 A) Boron is most commonly found as borax and boric acid, which are used in cleaning compounds. Aluminum is the third most common element in the earth's crust. It is used as a coating agent, to prevent oxidation. It is an excellent conductor of electricity and heat and can be found in many cooking utensils. Gallium is important today in the production of gallium arsenide LEDs and laser diodes. Indium is a very soft metal that can actually be wiped onto other metals as an anticorrosion agent. It also has the peculiar quality of squealing when bent. Finally, thallium is quite toxic and is sometimes used in rat poisons. It has also been used in glass to make special infrared filters.

Boron Family
Carbon Family p 2 (2 p 2 -6 p 2) n Elements in group 14 (4 A) n Contains elements important to life and computers. n Carbon is the basis for an entire branch of chemistry. n Silicon and Germanium are important semiconductors.

More about the carbon family n The element carbon is the basis of life. It is found in all living material. Silicon is a semiconductor used commonly in computer chips and solar cells. It is also the second most abundant element in the earth's crust. Silicon dioxide, Si. O 2, is the major component of glass. Germanium has important semiconductor properties and is used in the computer industry. It is one of the few elements that expand when frozen. Lead has long been used for plumbing and is also used to block radiation. Tin was once used to make cans because it is relatively stable -- unreactive. Aluminum has replaced the more expensive tin today.
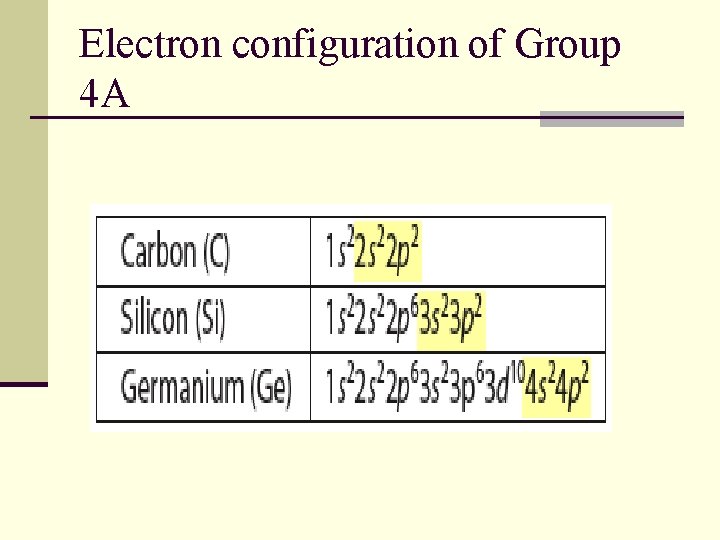
Electron configuration of Group 4 A

Carbon Family
Nitrogen Family p 3 (2 p 3 - 6 p 3) n Elements in group 15 (5 A) n Nitrogen makes up over ¾ of the atmosphere. n Nitrogen and phosphorus are both important in living things. n Most of the world’s nitrogen is not available to living things. n The red stuff on the tip of matches is phosphorus. n http: //www. carondelet. p vt. k 12. ca. us/Family/Scie nce/Nitrogen/thefamily. h tml

More about the nitrogen family n Nitrogen is used in saltpeter for fertilizer and explosives. It is also useful to create an oxygen-free atmosphere to prevent oxidation or combustion. A common use for liquid nitrogen today is the rapid freezing of food products. We also use liquid nitrogen in medical/surgical applications such as cryotherapy and cryosurgery. Phosphorus is used in compounds such as phosphoric acid, to make synthetic fertilizers, and in detergents. Arsenic and antimony are most commonly found in alloys used for the production of batteries and special types of solder. Bismuth is commonly used for alloys of metals and as a component of cosmetics or medicine used to treat upset stomach (Pepto-Bismol) and eczema.
Oxygen Family or Chalcogens p 4 2 p 4 - 6 p 4) n Elements in group 16 (6 A) n Oxygen is necessary for respiration. n Many things that stink, contain sulfur (rotten eggs, garlic, skunks, etc. )

The chalcogens (Oxygen family)

More about the oxygen family n Oxygen and sulfur are common elements. In fact, oxygen is the most common element (by mass) in the earth's crust. Because oxygen is second in electronegativity only to fluorine, it reacts with almost everything to form compounds here on earth. Selenium has some semimetal characteristics, such as an increase in electrical conductivity when a light is shined on it. Tellurium is a true semimetal, existing in compound with both positive and negative charges. Polonium is an extremely rare radioactive element discovered by Marie Curie and named for her native Poland. This means that the oxygen family is split between nonmetals and semimetals.
Halogens p 5 (2 p 5 - 6 p 5) n Elements in group 17 (7 A) n Very reactive, volatile, diatomic, nonmetals n Always found combined with other element in nature. n Used as disinfectants and to strengthen teeth.

The halogens

More about the halogens: n All of the elements of the halogen family are found in common use in everyday life. Fluorine is used in compounds to strengthen the enamel of your teeth against decay. It is also used in acid form to etch glass. Chlorine is used in our drinking water and in swimming pools to inhibit bacterial growth. It is also used in the form of chlorine dioxide to bleach wood pulp in the manufacture of very white paper. We also use chlorine in everyday laundry bleach. Chlorine compounds are used in insecticides, fireworks, and matches.

The representative elements (1 A-7 A) n Display a wide range of physical and chemical properties. n They are metals, nonmetals and metalloids. n At room temperature: most are solids, some are gases and one is a liquid. n The group number = number of electrons in outer energy level
1 A n The elements in the 1 A-7 a groups are called the representative 2 A elements 3 A 4 A 5 A 6 A 7 A 8 A 0

The Noble Gases n The noble gases are used in industry in arc welding, to dilute the oxygen in deep-sea divers' gas tanks, and to fill light bulbs. Argon is used in arc welding and in common light bulbs, as it does not react with the metal at high temperatures. Helium is used for diluting the pure oxygen in deep-sea diving tanks because the helium has a low solubility in human blood. Helium is also used to inflate the tires of large aircraft, weather balloons, and blimps because it is nonflammable. Neon is used in sign tubing because it glows bright red when electricity is passed through it. Krypton and xenon are used in photographic flash units and in lighthouses, as running an electric current through either element generates a very bright light.
The Noble Gases p 6 (2 p 6 -6 p 6) except for He =1 s 2 n Elements in group 18 n n (8 A) VERY unreactive, monatomic gases Used in lighted “neon” signs Used in blimps to fix the Hindenberg problem. Have a full valence shell.
The Noble Gases

More Noble Gases n Since all noble gases are colorless, odorless gases at room temperature, here are some pictures of how you might find them in use. n Xenon in headlights n Radon leaking into homes.
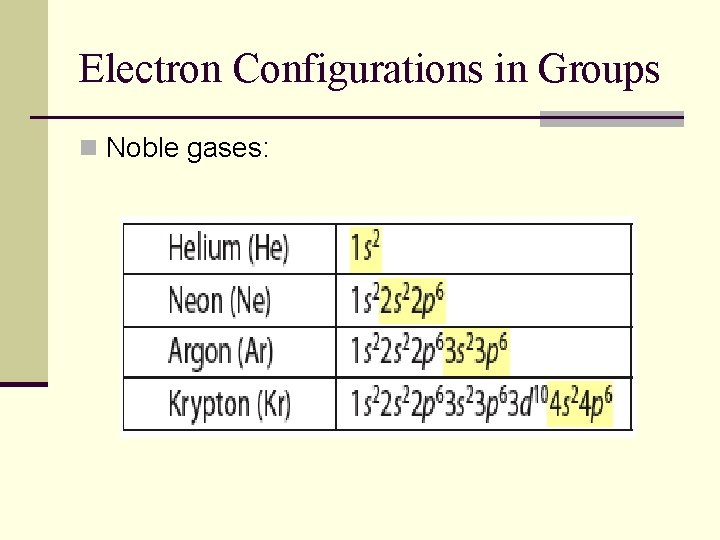
Electron Configurations in Groups n Noble gases:
Transition metals the “d” block inner transition metals the “f” block n Group 3 -12 (B)
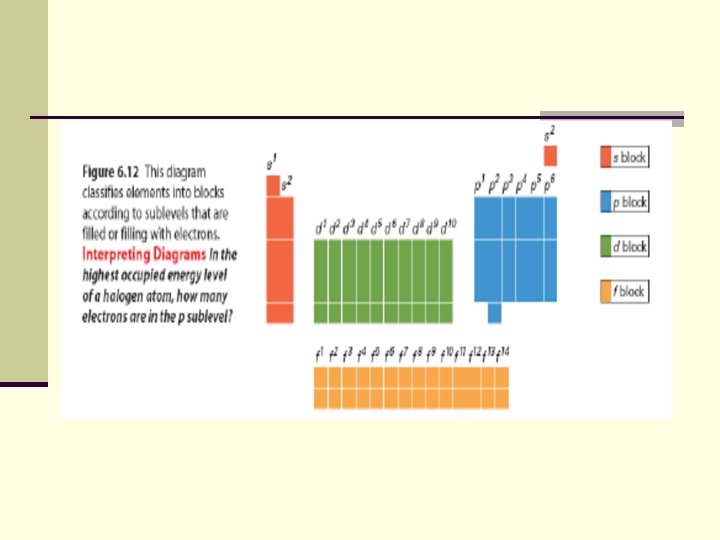
Periodic Trends Section 6. 3

Reasons for the trends: Pull of the nucleus on electrons vs. shielding effect of electrons in new energy levels

Pull of nucleus vs. electrons n. Increases across a period n. Decreases down a group
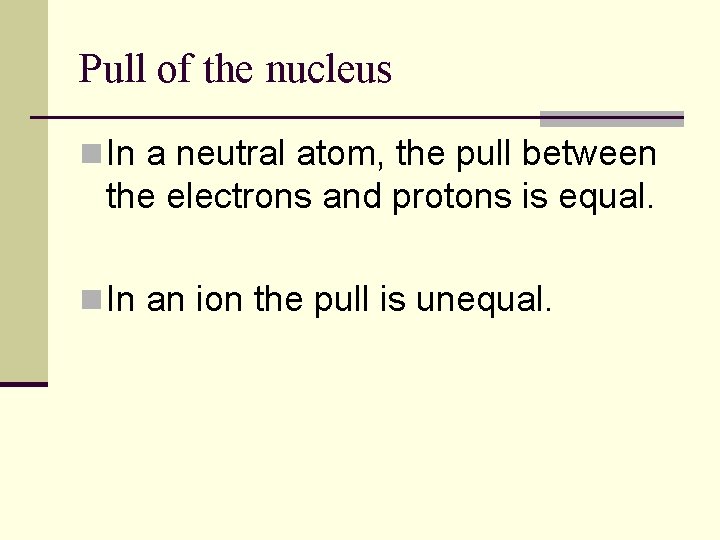
Pull of the nucleus n In a neutral atom, the pull between the electrons and protons is equal. n In an ion the pull is unequal.

Shielding effect n As the atomic number increases down a group, the energy level increase (Li, level 2, Cesium, level 6) so… n The increase in energy levels shields electrons from the pull of the nucleus.
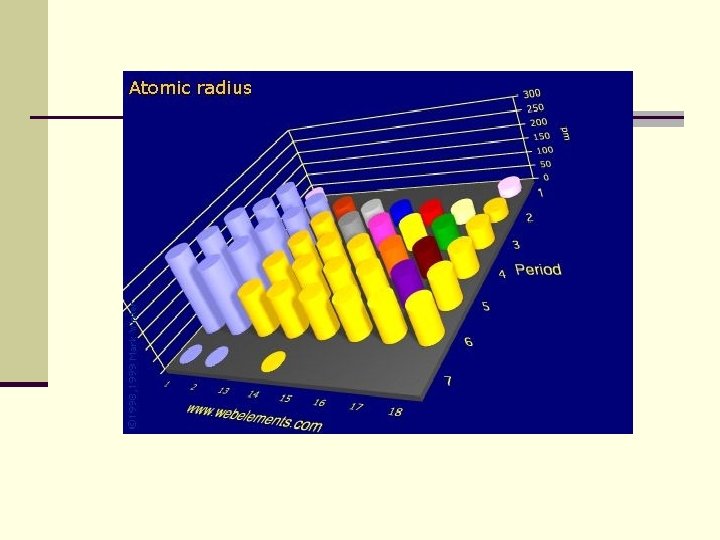
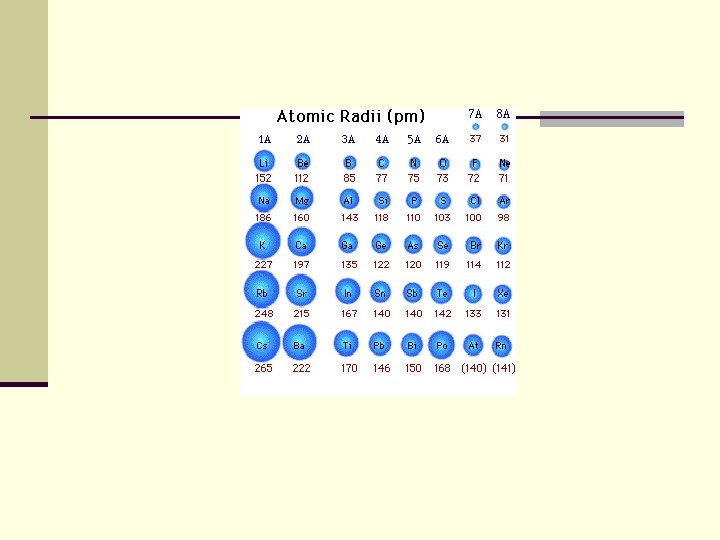
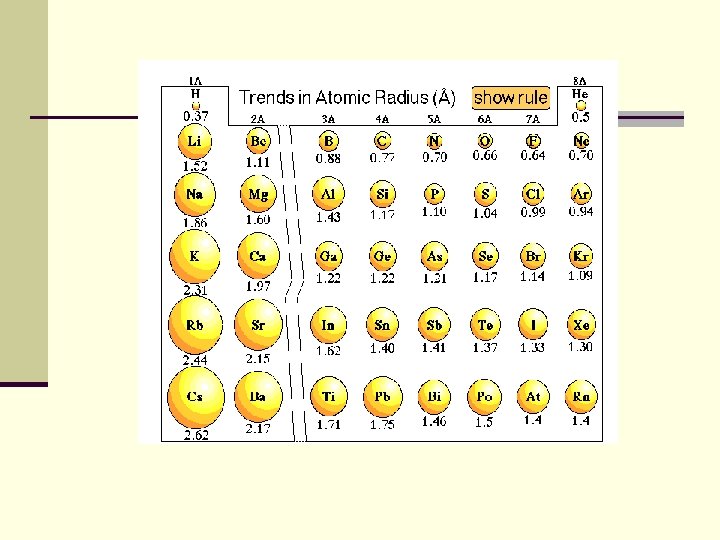

Atomic Radius decreases across a period n. Pull of the nucleus: There are more electrons and more protons within the same energy level=more pull n. Sheilding effect is not a factor

Atomic Radius increases down a group n. The shielding effect of more energy levels is greater than the increased pull of the nucleus on electrons (between more electrons and protons. )

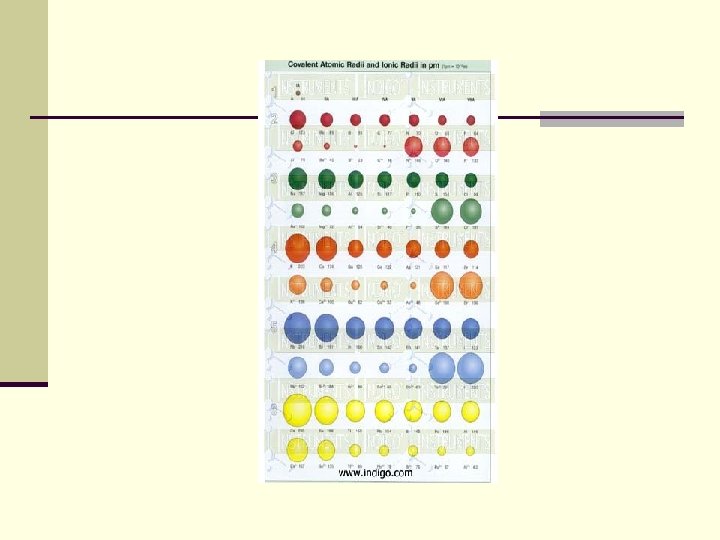
Ionic radius n Cations are always smaller than anions n Cations are smaller than their atom n Anions are larger than their atom

Ionic radius decreases across a period n Size of cations decreases n Size of anions decreases n More pull by the nucleus because there is no increase in shielding effect

Ionic radius generally increases down a group n. Because there is an increase in number of energy levels (shielding effect)

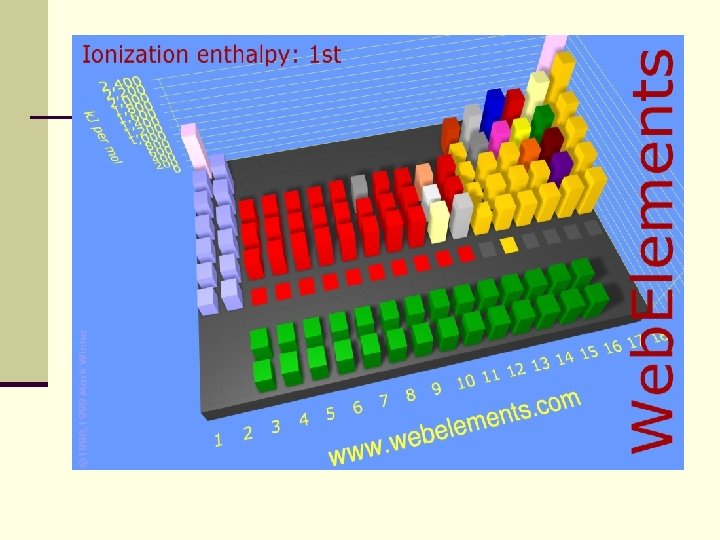
Ionization energy increases across a period n. There is a stronger pull between electrons and protons in the nucleus. n. The shielding effect is constant and therefore not a factor.

Ionization energy decreases down a group n Pull of the nucleus is minor n SHIELDING EFFECT is the major reason that atoms can release an electron easier
Electronegativity nthe ability of an atom of an element to attract electrons when an atom is in a compound

Electronegativity increases across a period n. Metals have low values because they have few electrons in outer energy level n and non-metals have high values because they need only a few electrons.

Electronegativity decreases down a group n. Cs (cesium)is lowest on table and F (fluorine) is highest

Electronegativity increases across a period n Pull of nucleus on electrons from other atoms is strong n Shielding effect does is not a factor

Electronegativity decreases down a group n Shielding effect is a factor on an atoms ability to pull in electrons from another atom n Increased pull from nucleus is not a major factor.
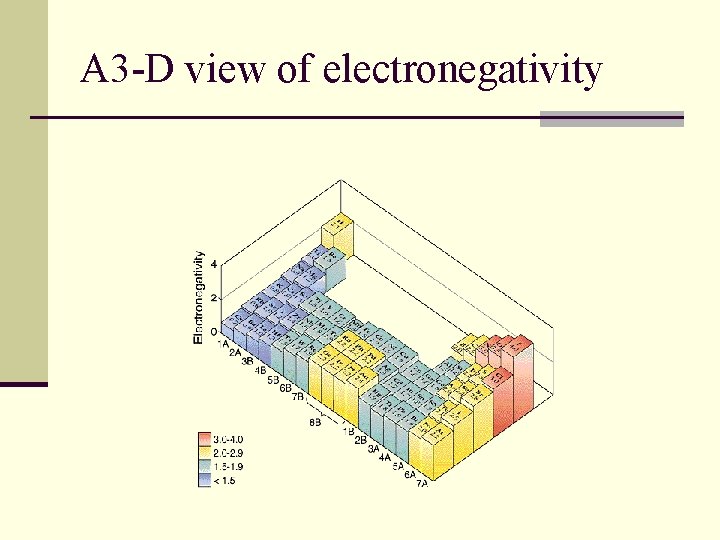
A 3 -D view of electronegativity

Credits: n http: //www. hcc. mnscu. edu/chem/V. 10/alkaline_earth_metals. jpg n http: //www. hcc. mnscu. edu/chem/V. 10/halogens. jpg n http: //images. google. com/imgres? imgurl=http: //www. learner. org/ interactives/periodic/images/boron. gif&imgrefurl=http: //www. lear ner. org/interactives/periodic/groups 9. html&h=157&w=294&sz=5 &hl=en&start=1&um=1&usg=__ow. IGrs. WIoy 8 VKmjb 6 HU 5 HRSDXA=&tbnid=kxa 37 e. LKn 0 NYc. M: &tbnh=61&tbnw=115& prev=/images%3 Fq%3 Dboron%2 Bfamily%26 um%3 D 1%26 hl%3 Den%26 rls%3 DTSHA, TSHA: 2004 -49, TSHA: en%26 sa%3 DN n http: //www. dkimages. com/discover/previews/ 796/939300. JPG
- Slides: 76