The Periodic Table Chapter 4 4 1 Mendeleevs

The Periodic Table Chapter 4
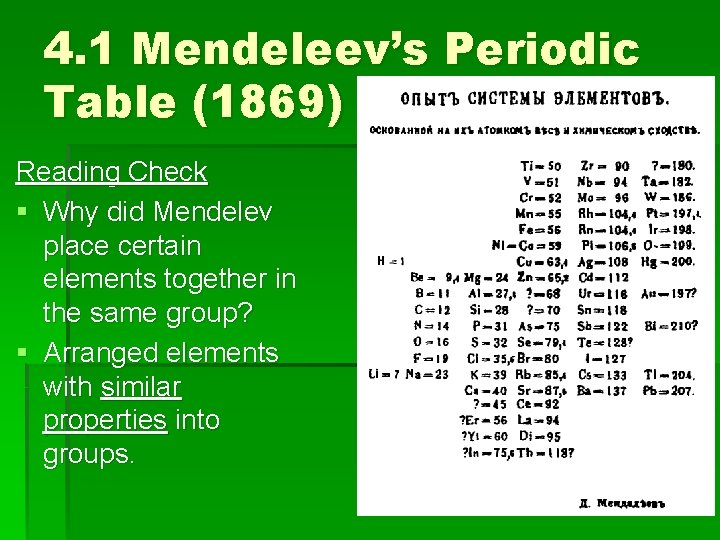
4. 1 Mendeleev’s Periodic Table (1869) Reading Check § Why did Mendelev place certain elements together in the same group? § Arranged elements with similar properties into groups.
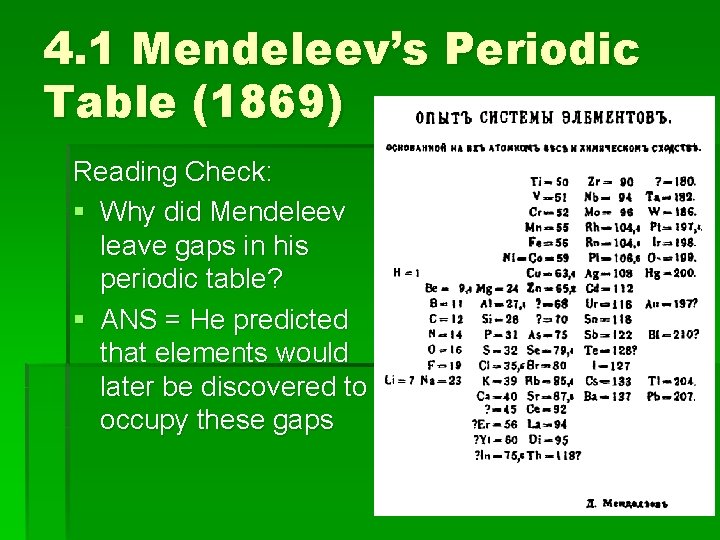
4. 1 Mendeleev’s Periodic Table (1869) Reading Check: § Why did Mendeleev leave gaps in his periodic table? § ANS = He predicted that elements would later be discovered to occupy these gaps
4. 1 Periodic Table § Why does the PT have the shape it does?

4. 1 Periodic Table § Elements are organized in order of increasing atomic number § Atomic mass follows the same order (in most cases)

4. 1 Periodic Table § Mendeleev did not know why certain elements have similar properties because he did not know about atomic theory. § But we do now! Why do properties repeat themselves? § The properties of an element are determined by it’s valence electrons.
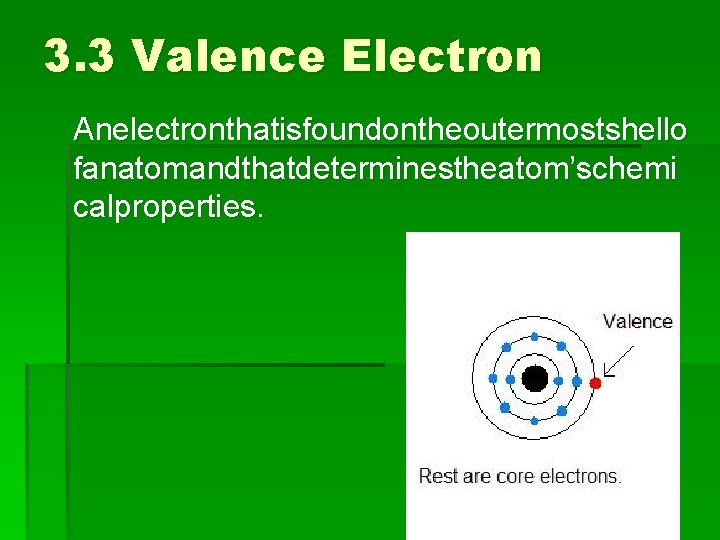
3. 3 Valence Electron Anelectronthatisfoundontheoutermostshello fanatomandthatdeterminestheatom’schemi calproperties.

3. 3 Valence Electron An electron that is found on the outermost shell of an atom and that determines the atom’s chemical properties. The electrons that are on the inside are called core electrons Which element is this?

Think about it…. . How many valence electrons are in there in a sulfur atom? (first, write the electron configuration)

Think about it…. . 1 s 2 2 p 6 3 s 2 3 p 4 2 -8 -6 How many valence electrons are in there in a sulfur atom? 6 valence electrons The rest are core electrons. How many core electrons? 10

4. 1 Valence electrons § How many valence electrons are there in a fluorine atom? § (first, write the electron configuration for fluorine)
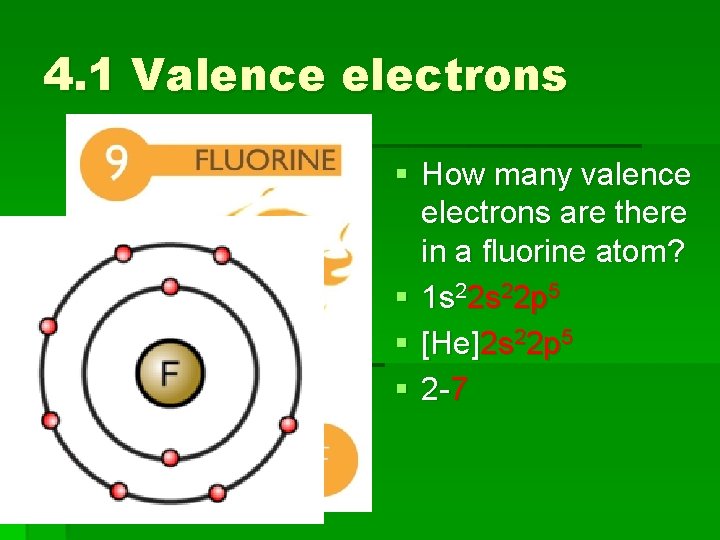
4. 1 Valence electrons § How many valence electrons are there in a fluorine atom? § 1 s 22 p 5 § [He]2 s 22 p 5 § 2 -7

4. 1 Periodic Table § A period (group/period) is a horizontal row in the periodic table § numbered 1 -7 § Corresponds to the energy level of the valence electrons

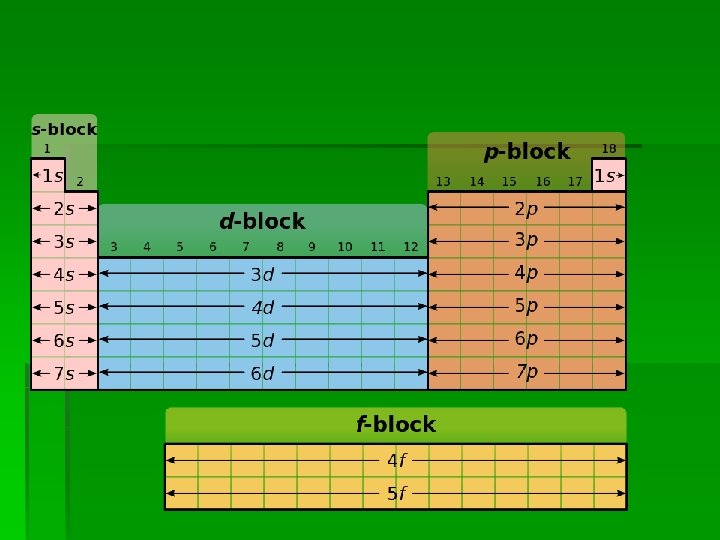
4. 1 Periodic Table A group is a vertical column in the periodic table ↓ Groups 1 -18 See fig 3 p 119 s-block, p-block, d-block, f-block

Think about it…. . 1. What is the name of the group of elements containing fluorine, chlorine, bromine and iodine? 2. How many valence electrons do these elements have? 3. What is the main characteristic of these elements? ANS= 1. The halogens 2. 7 valence electrons 3. High chemical reactivity
4. 3 Trends in the periodic table Standard Use the periodic table as a model to predict the relative properties of elements based on the patterns of electrons in the outer energy level of atoms. Learning Target Students learn about periodic trends in atomic radius by plotting/interpreting a graph

Think about it…. Atoms are small……but are they all the same size? Is a carbon atom the same size as an iron atom?
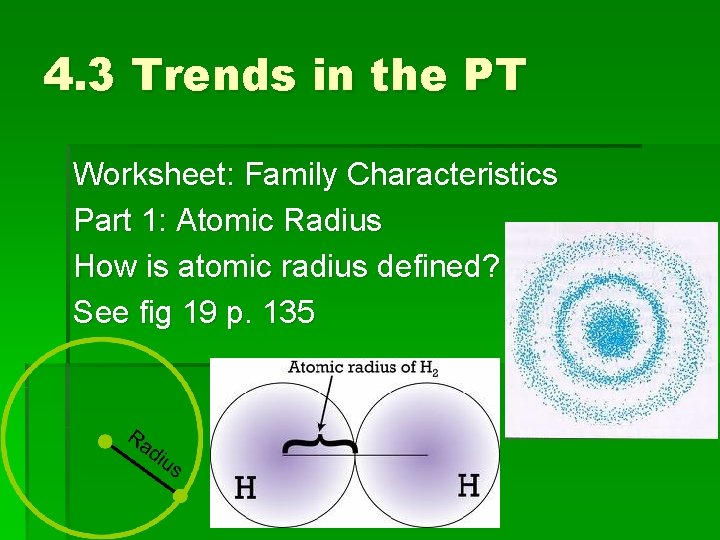
4. 3 Trends in the PT Worksheet: Family Characteristics Part 1: Atomic Radius How is atomic radius defined? See fig 19 p. 135

4. 3 Trend in Atomic Radius Draw in the lines for groups 2, 13, 14 & 17 on your worksheet. Inference: What is your graph telling you? What is the trend in atomic radius as you read down a group in the PT? What is the trend in atomic radius as you read across a period in the PT?

4. 3 Atomic Radius Summary See fig 20 p. 135, fig 21 p. 136 Inference: What are these diagram telling us? How are these diagrams similar/different from the one we plotted?

4. 3 Trends in the PT § How does atomic radius change as you read down a group in the PT? § ANS = Atomic radius increases (increases/decreases) as you read down a group in the PT.
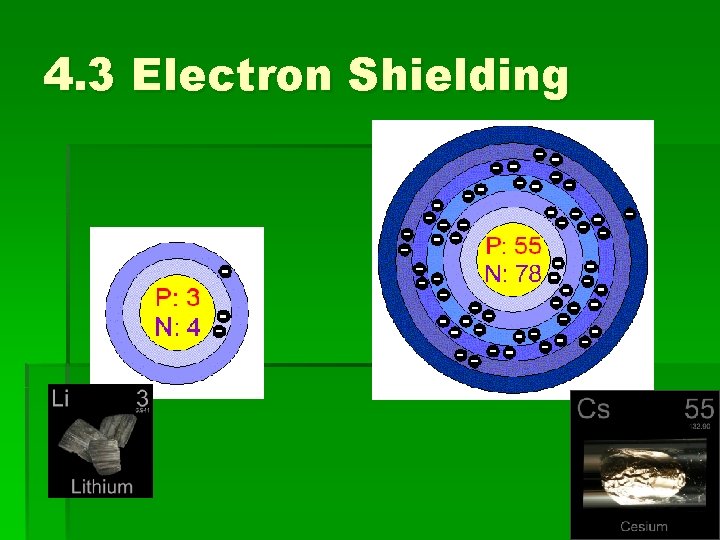
4. 3 Electron Shielding

4. 3 Trends in the PT § How does atomic radius change as you read across a period in the PT? § ANS = Atomic radius decreases (increases/decreases) as you read across a period in the PT.

4. 3 Trends in the PT Atomic radius decreases as you read across a period in the PT. COUNTERINTUITIVE Read p. 136 ‘Atomic Radius Decreases as You Move Across a Group’
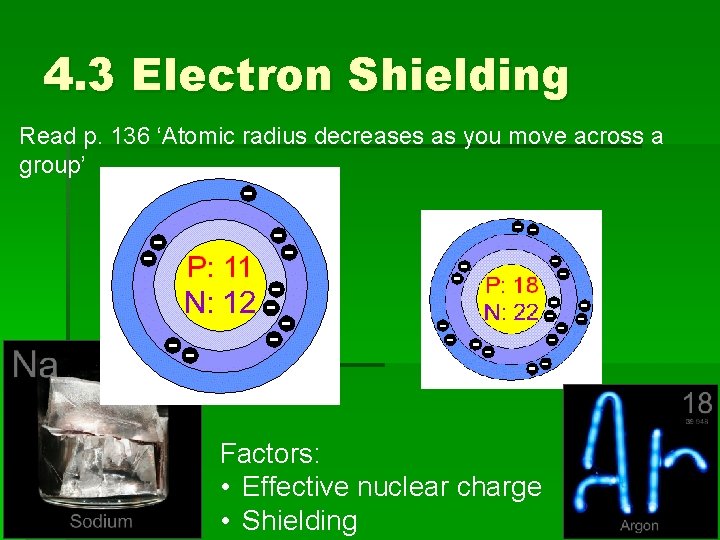
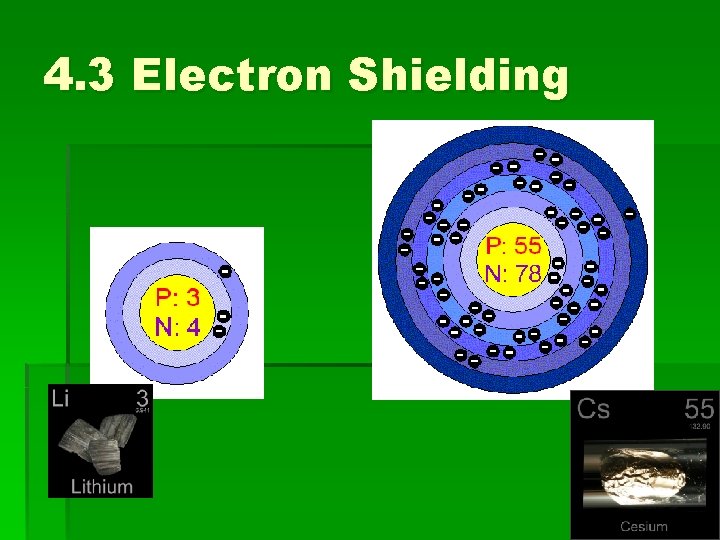
4. 3 Electron Shielding Read p. 136 ‘Atomic radius decreases as you move across a group’ Factors: • Effective nuclear charge • Shielding
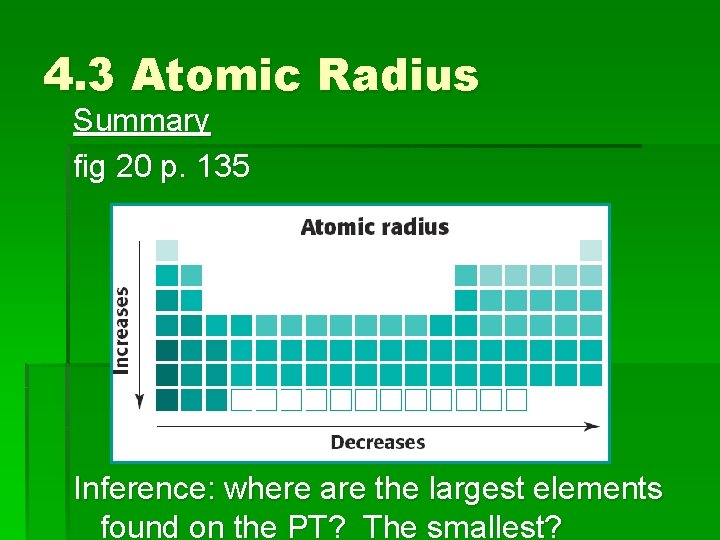
4. 3 Atomic Radius Summary fig 20 p. 135 Inference: where are the largest elements found on the PT? The smallest?
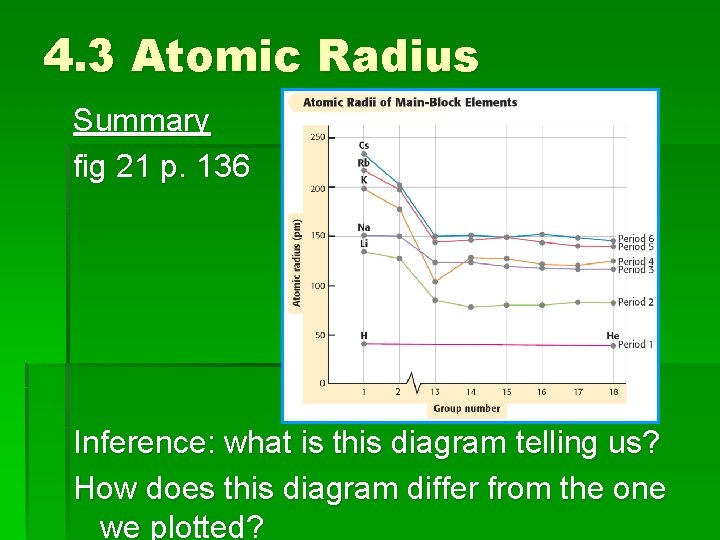
4. 3 Atomic Radius Summary fig 21 p. 136 Inference: what is this diagram telling us? How does this diagram differ from the one we plotted?
4. 3 Trends in the periodic table Standard Use the periodic table as a model to predict the relative properties of elements based on the patterns of electrons in the outer energy level of atoms. Learning Target Students learn about periodic trends in atomic radius by plotting/interpreting a graph
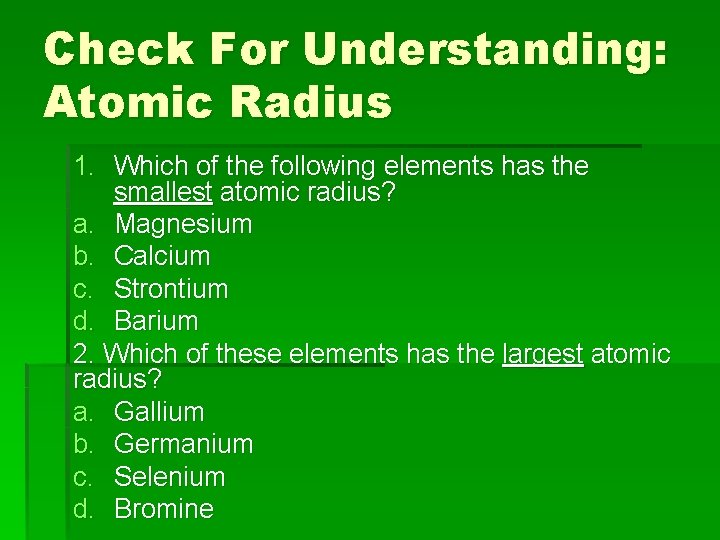
Check For Understanding: Atomic Radius 1. Which of the following elements has the smallest atomic radius? a. Magnesium b. Calcium c. Strontium d. Barium 2. Which of these elements has the largest atomic radius? a. Gallium b. Germanium c. Selenium d. Bromine

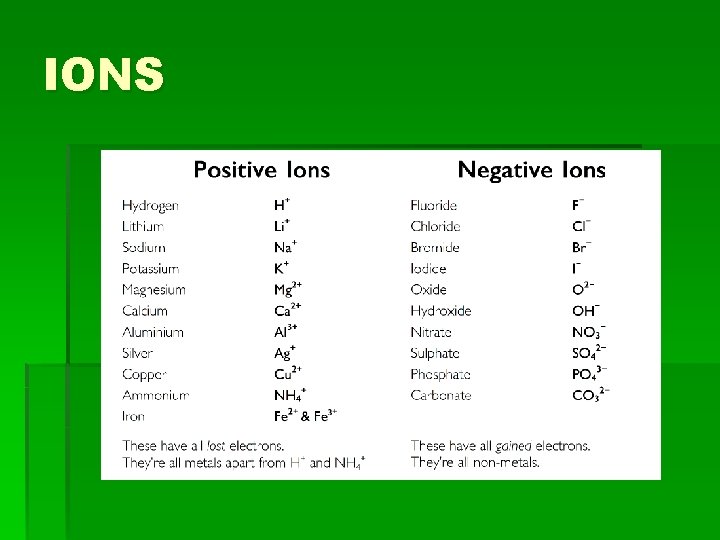
4. 3 Ions Notes: • Whenatomshaveequalnumbersof protonsandelectronstheyareelect ricallyneutral

4. 3 Ions • When atoms have equal numbers of protons and electrons they are electrically neutral. • Butwhenenoughenergyisadded, t heattractiveforcecanbeovercome.

4. 3 Ions • But when enough energy is added, the attractive force can be overcome. • Whenthishappens, anelectronisre movedfromtheatom, anditbecome sapositivelychargedion

4. 3 Ions • When this happens, an electron is removed from the atom, and it becomes a positively charged ion. (p. 133) Positively charged ion = ‘cation’
IONS
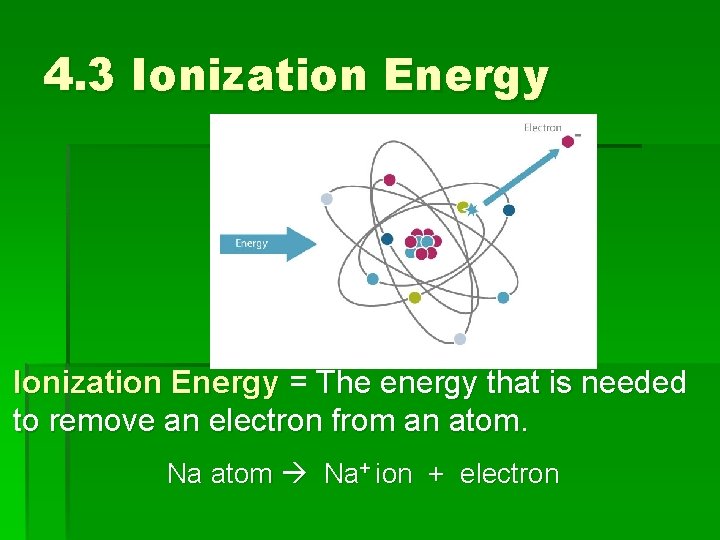
4. 3 Ionization Energy = Energy The energy that is needed to remove an electron from an atom. Na atom Na+ ion + electron
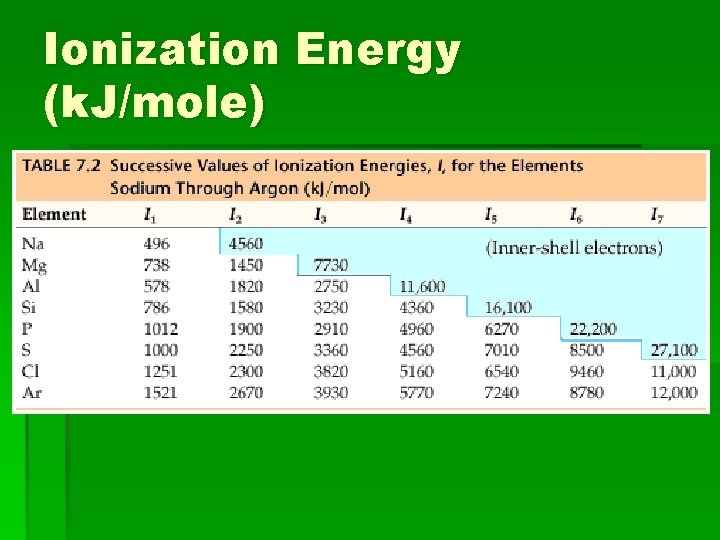
Ionization Energy (k. J/mole)

4. 3 Ionization energy If ionization energy is high, it is difficult (difficult/easy) to remove an electron from the atom. Ionization energy is related to chemical reactivity.

4. 3 Trend in ionization energy Read Family Characteristics worksheet Part 2 Draw in the lines for groups 1, 13, 15, 17, 18 on your worksheet. Inference: What is your graph telling you?

4. 3 Ionization Energy § Ionization Energy decreases (increases or decreases) as you read down a group in the PT. § Ionization Energy increases (increases/decreases) as you read across a period in the PT

Think about it…. . § In the Brainiac alkali metals video clip, which group 1 element was the most reactive when put into water? ANS = Those at the bottom Rb & Cs

4. 3 Ionization Energy Read p. 133 ‘Ionization Energy Decreases As You Move Down a Group’
4. 3 Electron Shielding

4. 3 Effective Nuclear Charge Read Ionization Energy Increases as you Move Across a Period p. 134
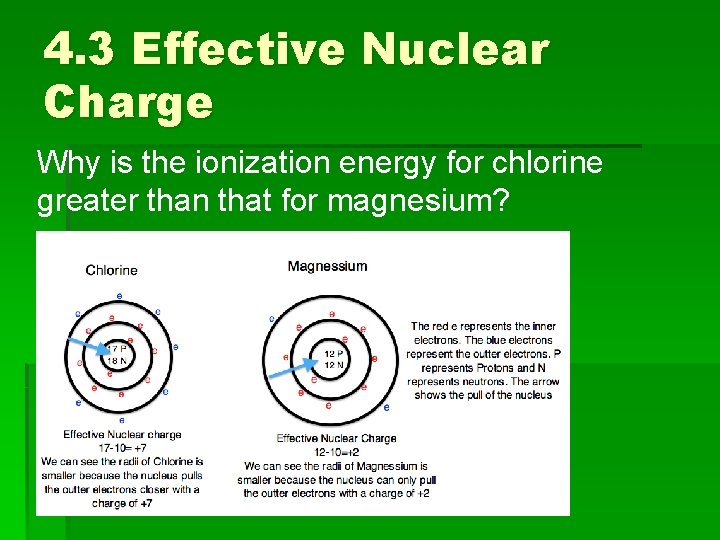
4. 3 Effective Nuclear Charge Why is the ionization energy for chlorine greater than that for magnesium?
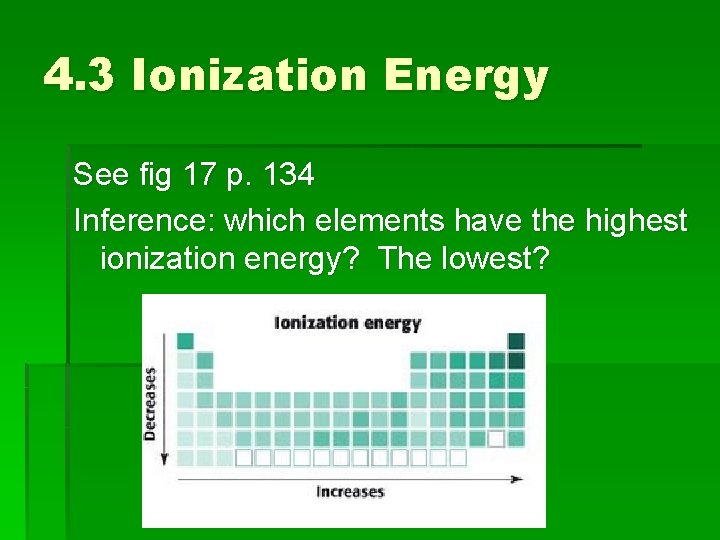
4. 3 Ionization Energy See fig 17 p. 134 Inference: which elements have the highest ionization energy? The lowest?

Periodic Trends What is the relationship between atomic radius and ionization energy? An atom with a small atomic radius has a high (high/low) ionization energy. e. g. Fluorine
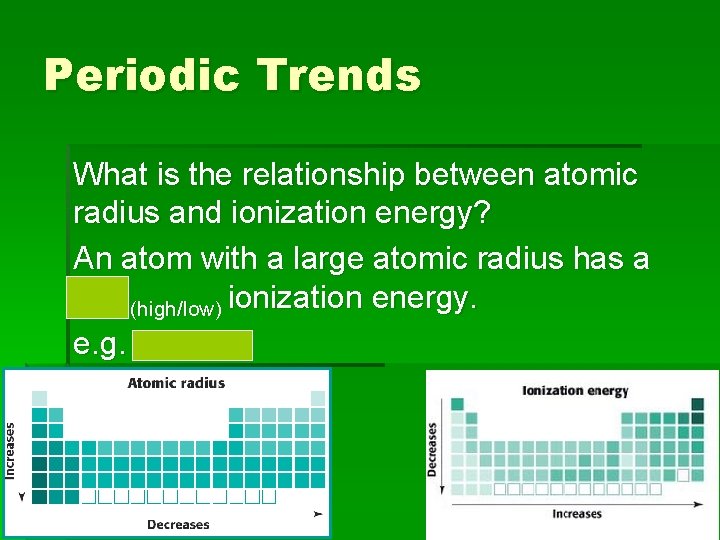
Periodic Trends What is the relationship between atomic radius and ionization energy? An atom with a large atomic radius has a low (high/low) ionization energy. e. g. Cesium

Think about it…. 1. Which element has the largest atomic radius: Mg or Ca? 2. Which element has the largest atomic radius: Al or S? 3. Which element has the largest ionization energy: Mg or Ca? ANS = 1. Ca, b/c it has more shells of electrons than Mg 2. Al, b/c it has a lower nuclear charge than S 3. Mg, b/c it has a smaller atomic radius and therefore holds it’s electrons more tightly.

Electronegativity Definition: Ameasureoftheabilityofanatominachemical compoundtoattractelectronstoitself. A measure of the ability of an atom in a chemical compound to attract electrons to itself. (p. 137) Facts: Read/Notes ‘Electronegativity’ p. 137 (first 3 paragraphs)
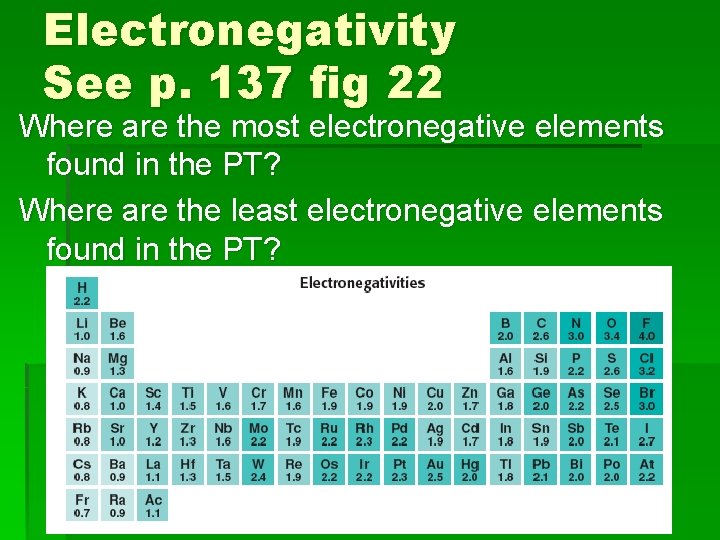
Electronegativity See p. 137 fig 22 Where are the most electronegative elements found in the PT? Where are the least electronegative elements found in the PT?
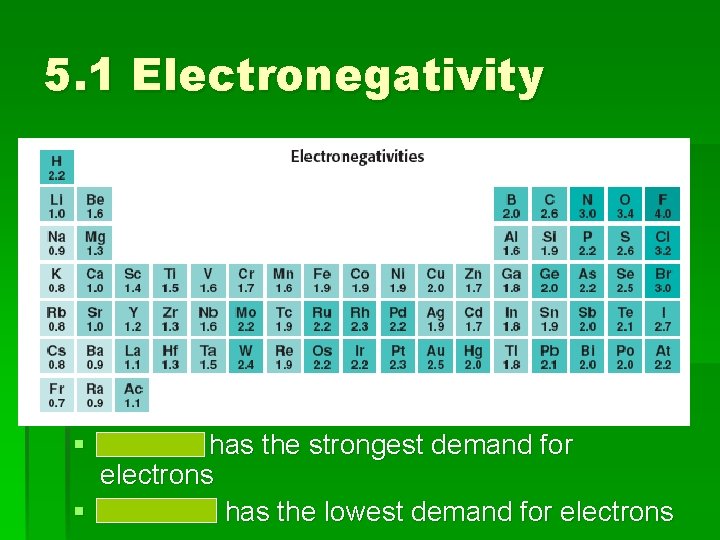
5. 1 Electronegativity § Fluorine has the strongest demand for electrons § Francium has the lowest demand for electrons
Periodic Trends What is the relationship between ionization energy and electronegativity? An atom with a high ionization energy has a high (high/low) electronegativity e. g. Fluorine Directly proportional
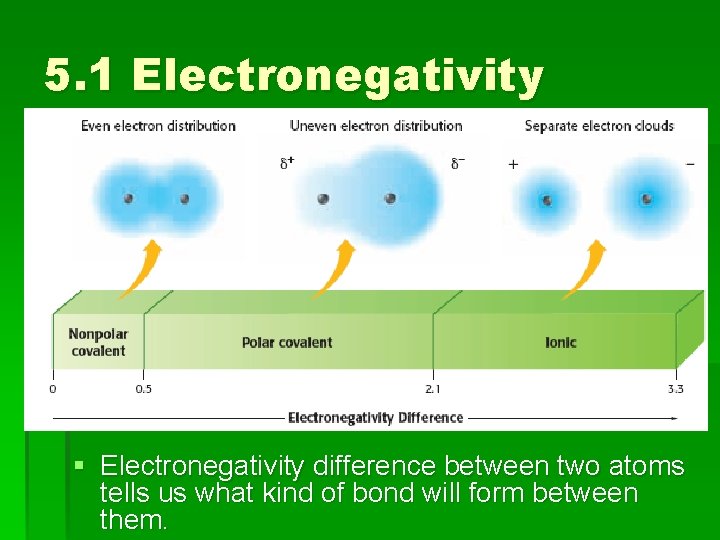
5. 1 Electronegativity § Electronegativity difference between two atoms tells us what kind of bond will form between them.
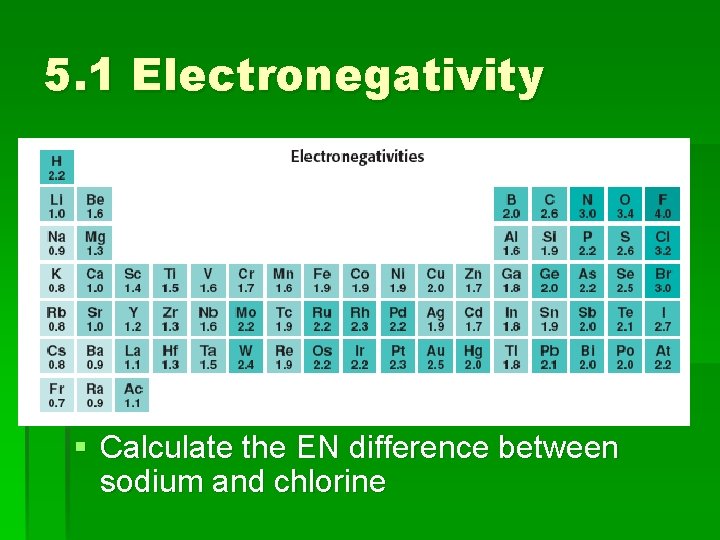
5. 1 Electronegativity § Calculate the EN difference between sodium and chlorine
- Slides: 57