The Periodic Table and Periodicity Arrangement n In

The Periodic Table and Periodicity
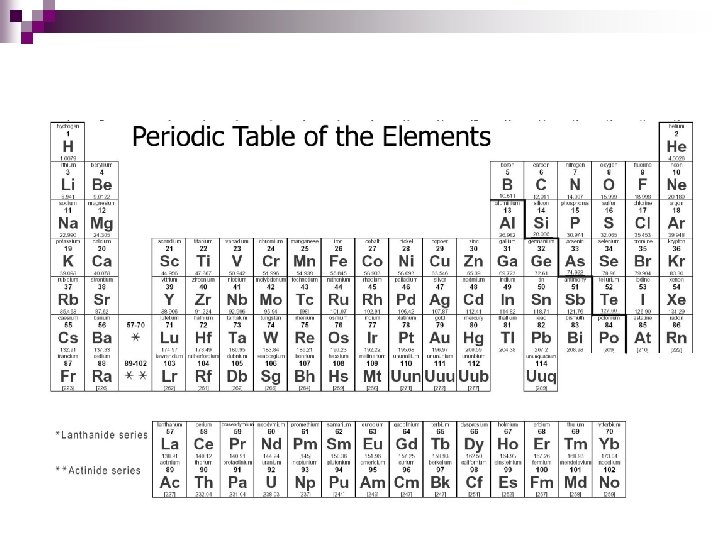

Arrangement n In order of increasing atomic number in specific columns and rows.
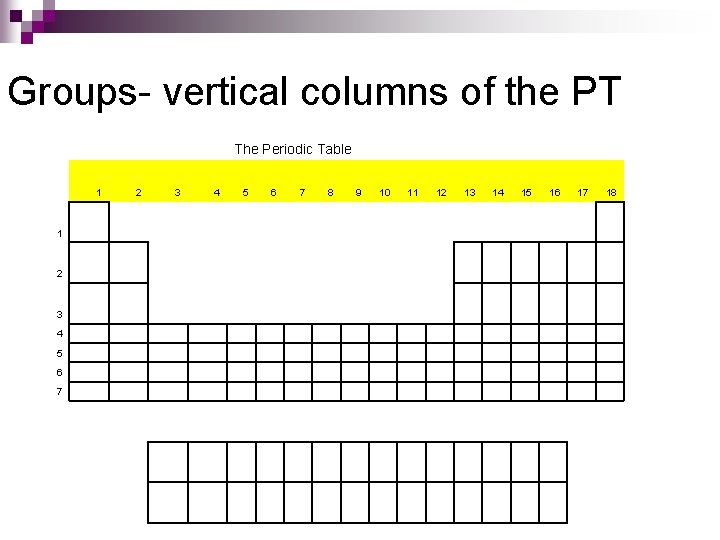
Groups- vertical columns of the PT The Periodic Table 1 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
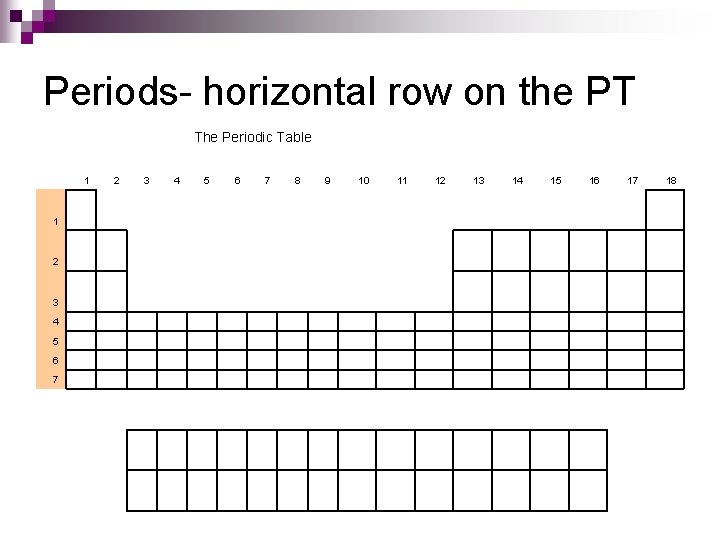
Periods- horizontal row on the PT The Periodic Table 1 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18

Groups are important on the PT n Why? ¨ The elements in a group have similar chemical and physical properties!
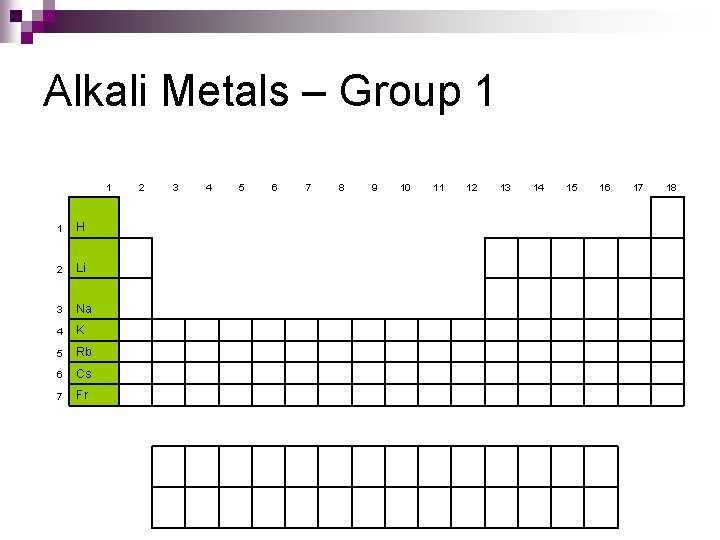
Alkali Metals – Group 1 1 1 H 2 Li 3 Na 4 K 5 Rb 6 Cs 7 Fr 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18

Alkaline Earth Metals – Group 2 1 2 Be 3 Mg 4 Ca 5 Sr 6 Ba 7 Ra 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
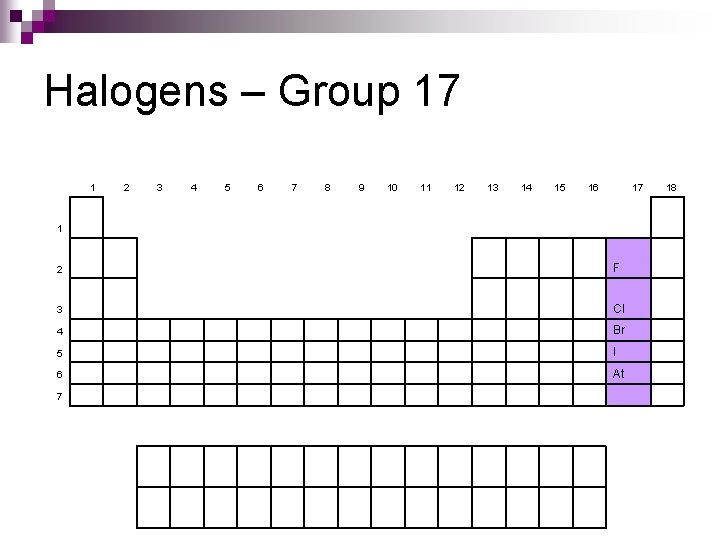
Halogens – Group 17 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 1 2 F 3 Cl 4 Br 5 I 6 At 7 18
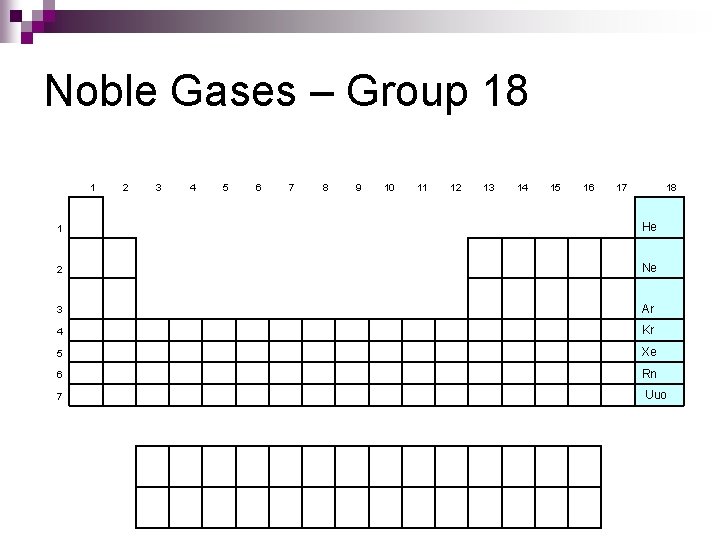
Noble Gases – Group 18 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 1 He 2 Ne 3 Ar 4 Kr 5 Xe 6 Rn 7 Uuo
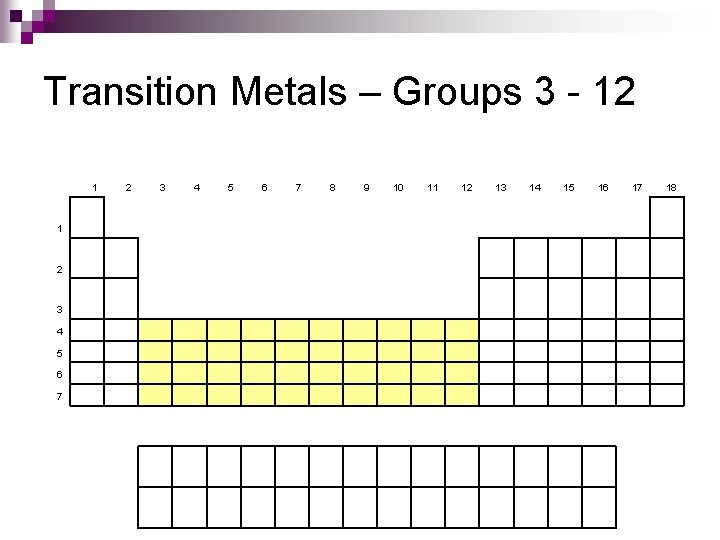
Transition Metals – Groups 3 - 12 1 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
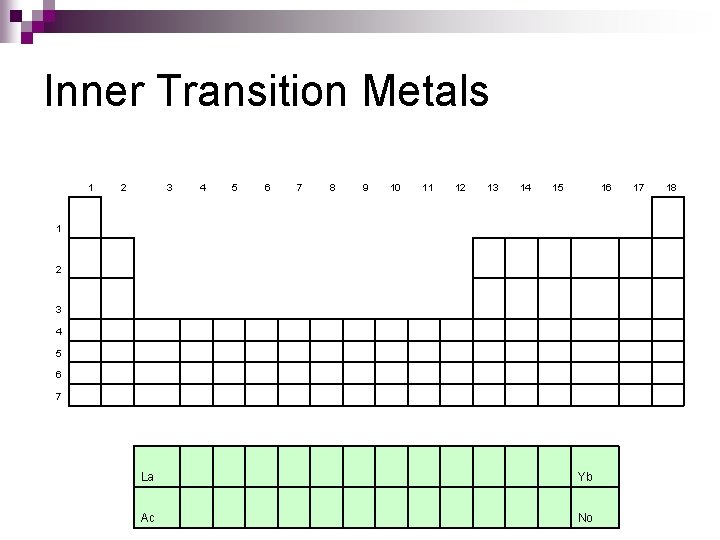
Inner Transition Metals 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 1 2 3 4 5 6 7 La Yb Ac No 17 18

Metals Lustrous n Good conductors of heat & electricity n Malleable – can be pounded into thin sheets n Ductile – can be drawn into thin wire n
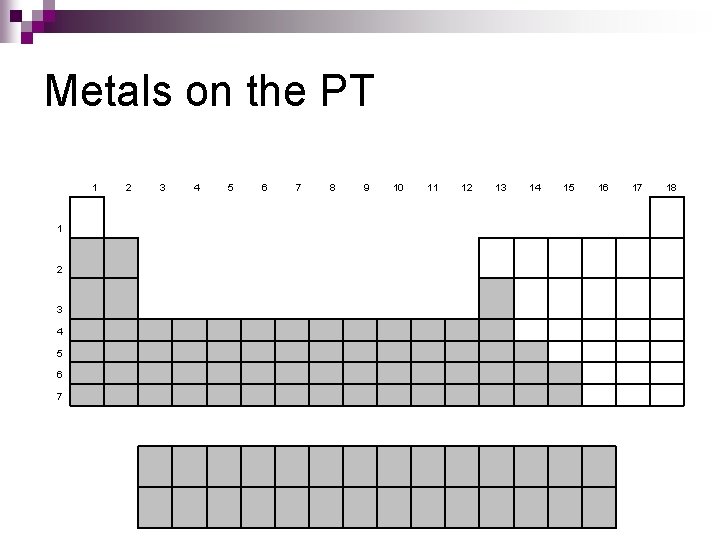
Metals on the PT 1 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
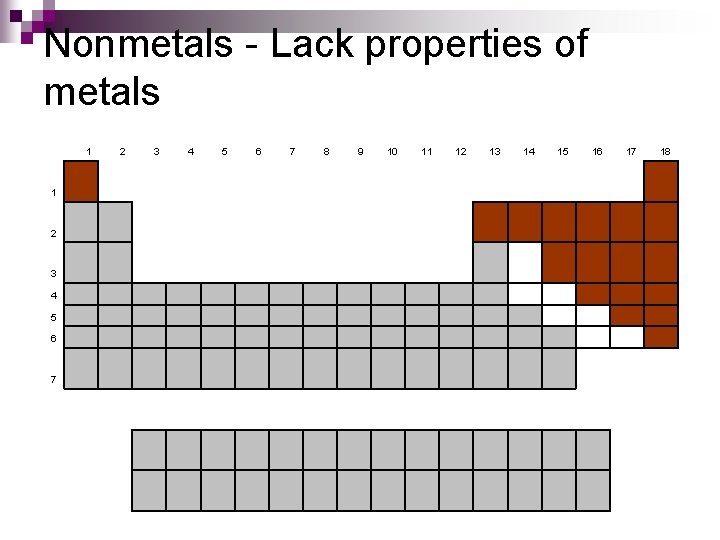
Nonmetals - Lack properties of metals 1 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
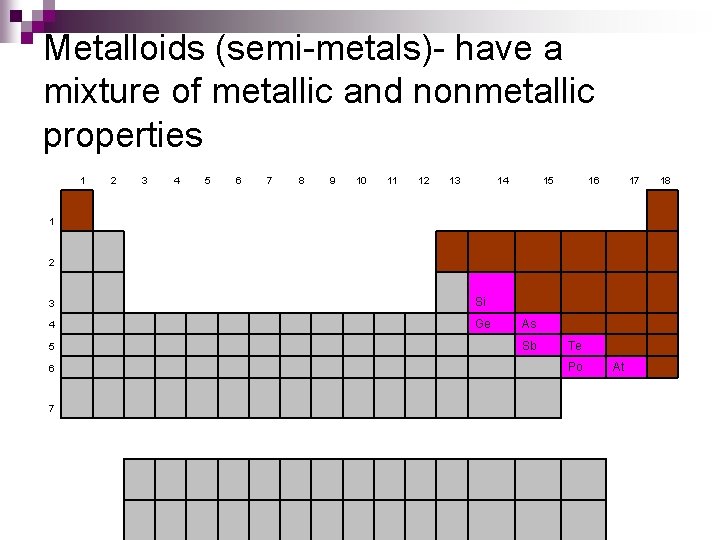
Metalloids (semi-metals)- have a mixture of metallic and nonmetallic properties 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 1 2 3 Si 4 Ge 5 6 7 As Sb Te Po At 18
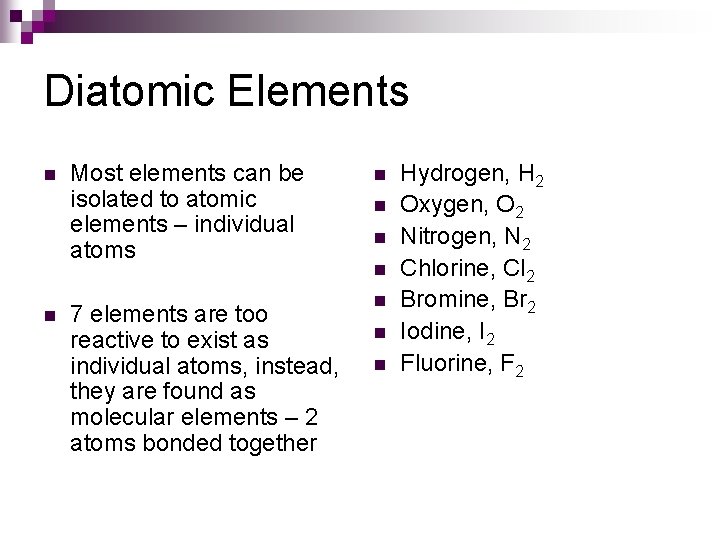
Diatomic Elements n n Most elements can be isolated to atomic elements – individual atoms 7 elements are too reactive to exist as individual atoms, instead, they are found as molecular elements – 2 atoms bonded together n n n n Hydrogen, H 2 Oxygen, O 2 Nitrogen, N 2 Chlorine, Cl 2 Bromine, Br 2 Iodine, I 2 Fluorine, F 2
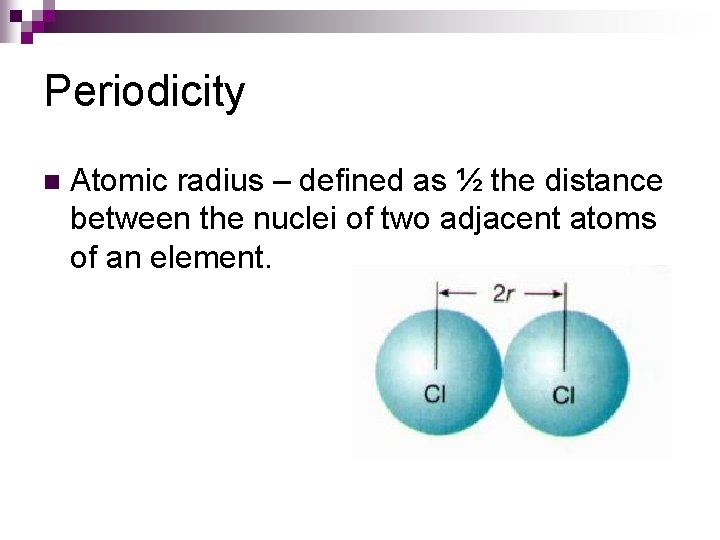
Periodicity n Atomic radius – defined as ½ the distance between the nuclei of two adjacent atoms of an element.

Periodicity n First Ionization Energy – energy needed to remove an electron from an atom. n Electronegativity – ability of an atom to attract electrons to itself
- Slides: 19