The Periodic Table And how to find the
The Periodic Table …And how to find the number of protons, neutrons & electrons in ANY atom!

ASAP Science Element Song § https: //www. youtube. com/watch? v=Vg. VQKCcfwn. U
The Periodic Table Elements are organized based on their physical and chemical properties in the periodic table: § Elements listed in rows by increasing atomic number § Rows are called periods § Columns are called families or groups § Elements with similar properties line up in vertical columns
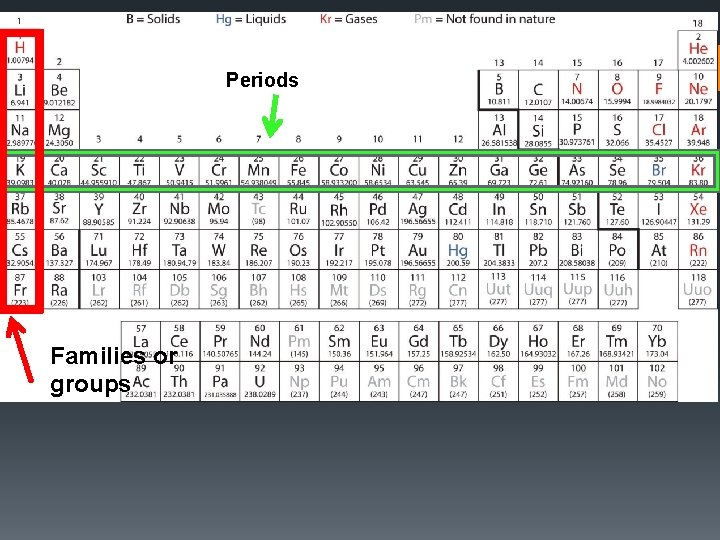
Periods Families or groups

Dmitri Mendeleev § Godfather of the periodic table § 1 st guy smart enough to record important properties of known metals and find a way to organize it § Also considered elements that had not been discovered yet.

VIDEO
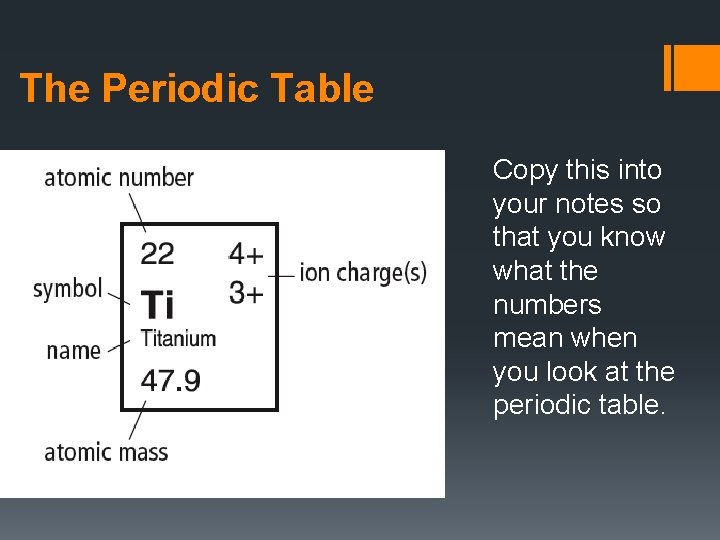
The Periodic Table Copy this into your notes so that you know what the numbers mean when you look at the periodic table.
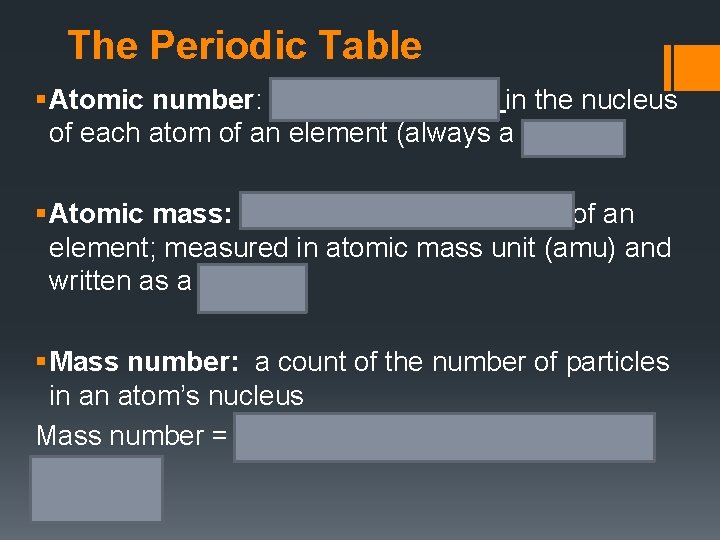
The Periodic Table § Atomic number: number of protons in the nucleus of each atom of an element (always a whole #) § Atomic mass: mass of an average atom of an element; measured in atomic mass unit (amu) and written as a decimal § Mass number: a count of the number of particles in an atom’s nucleus Mass number = (number of protons) + (number of neutrons)

How to find the number of protons, electrons and neutrons 1. The number of protons is… § # of protons = ATOMIC NUMBER 2. The number of electrons is… § Atoms have a neutral charge so they must have an equal number of protons and electrons § THEREFORE… Atomic number = # of protons = # of electrons

3. The number of neutrons is… § Mass number = (number of protons) + (number of neutrons) § Mass number is not given in the periodic table BUT. . . all you need to do is round the atomic mass to the nearest whole number
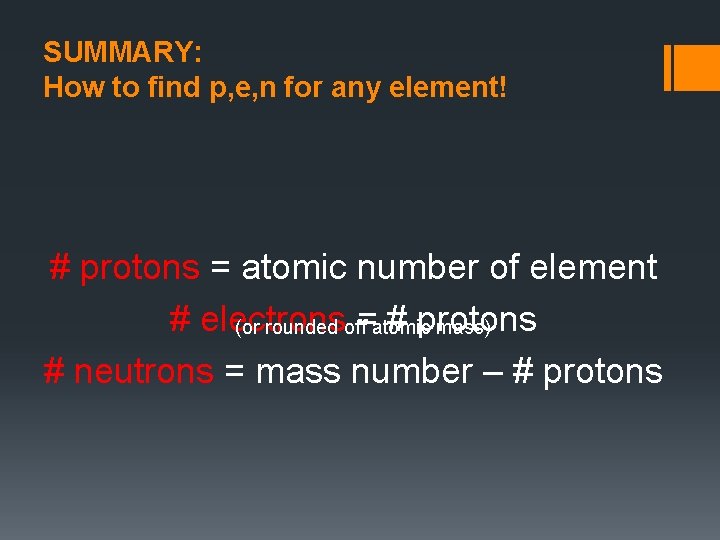
SUMMARY: How to find p, e, n for any element! # protons = atomic number of element # electrons = # protons (or rounded off atomic mass) # neutrons = mass number – # protons
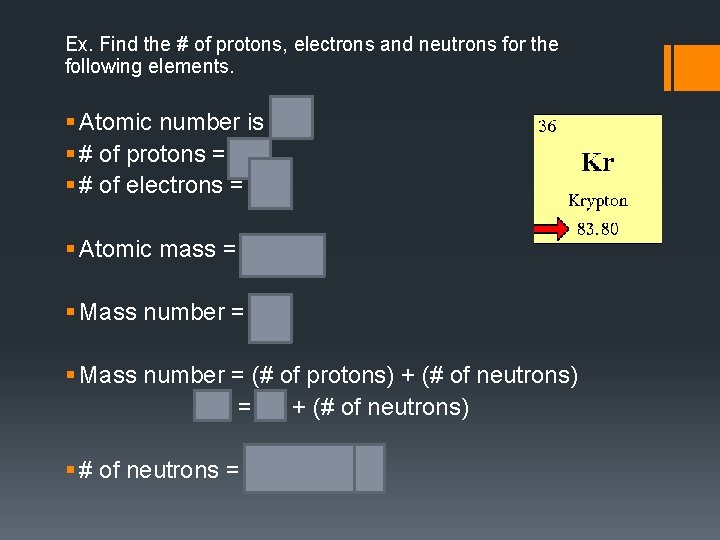
Ex. Find the # of protons, electrons and neutrons for the following elements. § Atomic number is 36 § # of protons = 36 § # of electrons = 36 § Atomic mass = 83. 80 § Mass number = 84 § Mass number = (# of protons) + (# of neutrons) 84 = 36 + (# of neutrons) § # of neutrons = 84 – 36 = 48
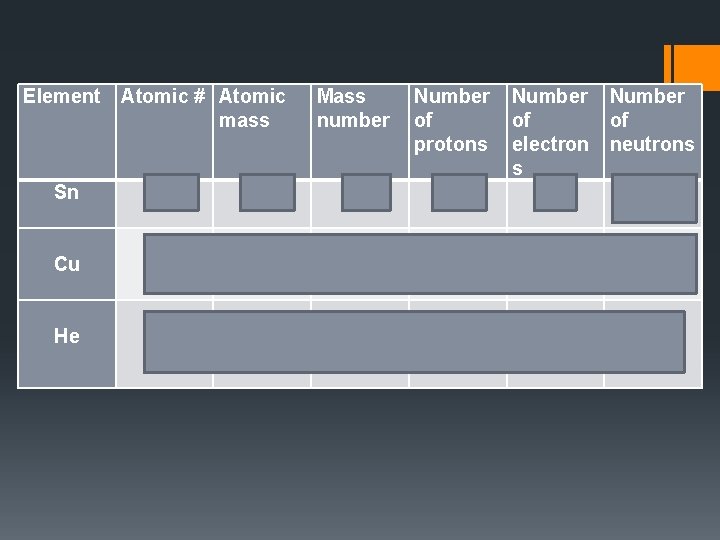
Element Atomic # Atomic mass Sn 50 118. 7 119 Cu 29 63. 5 64 He 2 4 4 Mass number Number of of of protons electron neutrons s 50 50 119 -50= 69 29 29 64 -29 = 35 2 2 4 -2 = 2
Charges Listed on the Periodic Table § Ion charge: an electric charge that forms when an atom gains or loses electrons § This is listed in the Periodic Table § Ion: a charged atom § If an atom loses electron(s) positive charge (lost negative charge) § If an atom gains electron(s) negative charge (gains negative charge)
- Slides: 14