The Periodic Table and Atomic Structure Section 2
The Periodic Table and Atomic Structure Section 2. 1

Review � Last class? � Four classical atomic models � Quantum Mechanical Model
Objectives � distinguish between metals, non-metals, and metalloids in terms of distinguishing properties � explain the structure of the periodic table � identify important families and elements � describe atomic theory in terms of subatomic particles and location (nucleus, energy levels) � define atomic number, mass number, isotope, and atomic molar mass and be able to identify these for a given element � determine numbers of protons, neutrons and electrons for a given element
The Elements � 115: basic building blocks � 90 naturally occurring, 25 synthetic � Split into 3 classes ◦ Metals ◦ Non-metals ◦ metalloids
Metals � Most elements � Silver/grey and shiny � Conductors of heat and electricity � Malleable and ductile � Most solid at room temperature � Reactivity varies ◦ Inert= unreactive
Non-Metals � 17 elements � Grouped based on their different from metals � Vary is state, color and reactivity ◦ Highly reactive= fluorine ◦ Inert= noble gases � About half will appear at molecules (more than one atom)
Metalloids � Remaining elements � Properties intermediate between metals and non-metals � Along the staircase (except aluminium) � Ex. Silicon, arsenic, boron
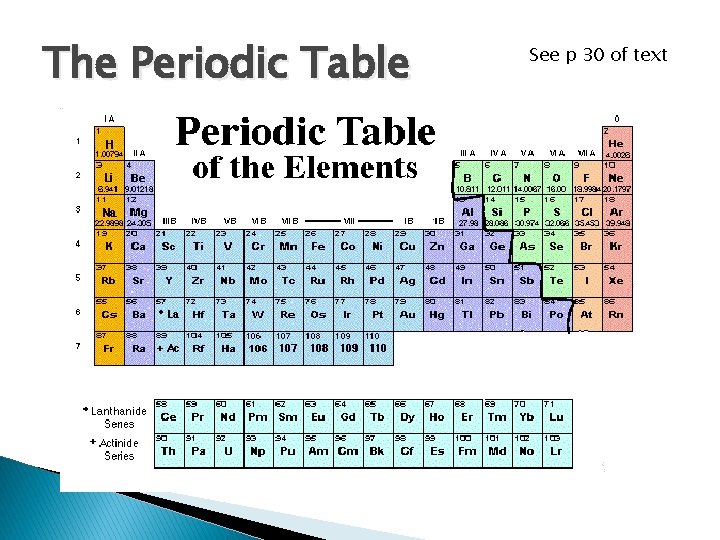
The Periodic Table See p 30 of text
The Periodic Table � Organizes properties elements based on chemical ◦ Metals on left side and centre ◦ Non-metals on far right �Exception hydrogen (learn more later) ◦ Metalloids between two � Shows name and symbol for element ◦ Get to know the symbols! http: //periodictable. com/

The Element Game � For the following, identify as being a metal, non-metal or metalloid � Hydrogen � Sulfur � Calcium � Tin � Gold � Boron � Aluminium � xenon

Pre-test � For the following, give the element’s name or symbol: ◦ ◦ ◦ ◦ Calcium Hydrogen Sulfur Phosphorus Mg Na Cl Br

Important Families � Groups of elements with similar chemical/physical properties � Group 1: alkali metals ◦ Soft, shiny, silver, very reactive with water ◦ Compounds white and soluble in water � Group 2: alkali earth metals ◦ Shiny, silver ◦ White compounds but not as soluble � Groups 3 -12: transition metals ◦ Shiny, most are silver ◦ Properties and electron structure varies (no pattern)
Important Families cont… � Group 16: chalcogens (or oxygen group) � Group 17: halogens ◦ Non-metals (smaller ones) & metals (larger ones) ◦ reactive ◦ Non-metals ◦ Very reactive �React with alkali metals to make salts � Group 18: noble gases ◦ Non-metals ◦ Very unreactive
Atomic Theory � Atoms are very small (10 -10 m in diameter) � What are three kinds of subatomic particles? Where are they found? � Protons and neutrons are over 99. 9% of total mass ◦ Imagine this: our classroom filled with iron. If we take out the nuclei for all the iron atoms and place them side by side, they would be as big as a period in a book. But that period would almost equal the mass of the room full of iron

Atomic Number � Number of protons � Determines the element Can you find the atomic number of the following elements? Pb Hg tin chlorine platinum Cu manganese lithium As we move through the periodic table, what do you notice about the ordering of atomic numbers?
Mass Number and Isotopes � Isotope- atoms of same element with different number of neutrons ◦ Ex. Common form of hydrogen has one proton and no neutrons. 1/10 000 hydrogen atoms have a neutron, called deuterium (“heavy” hydrogen) � Each isotope given a mass number ◦ Equals total number of protons and neutrons (don’t include electrons- too small) ◦ # of neutrons= mass number – atomic number
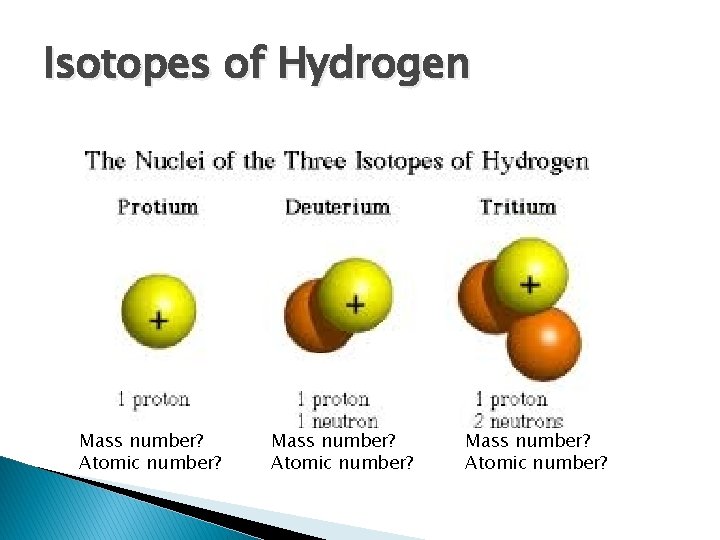
Isotopes of Hydrogen 1 H 1 Mass number? Atomic number? 2 1 H Mass number? Atomic number? 3 H 1 Mass number? Atomic number?
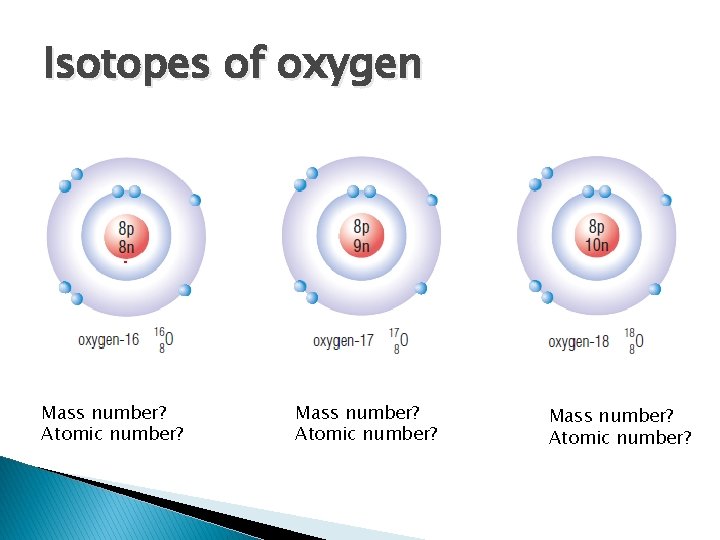
Isotopes of oxygen Mass number? Atomic number?
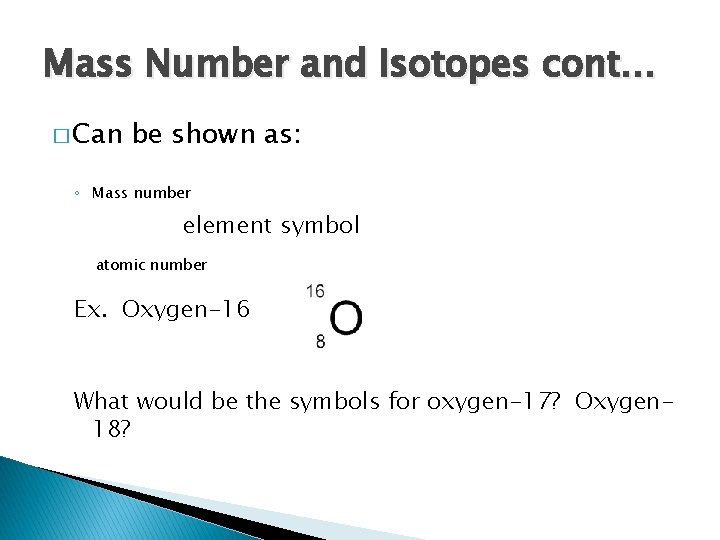
Mass Number and Isotopes cont… � Can be shown as: ◦ Mass number element symbol atomic number Ex. Oxygen-16 What would be the symbols for oxygen-17? Oxygen 18?
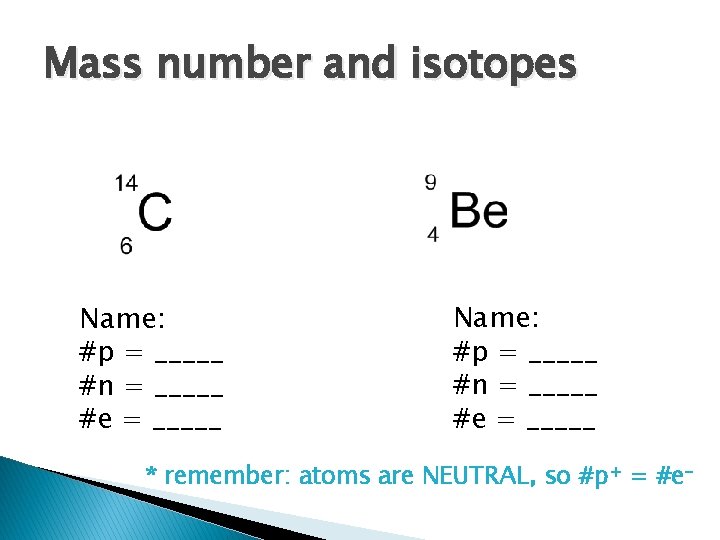
Mass number and isotopes Name: #p = _____ #n = _____ #e = _____ * remember: atoms are NEUTRAL, so #p+ = #e-
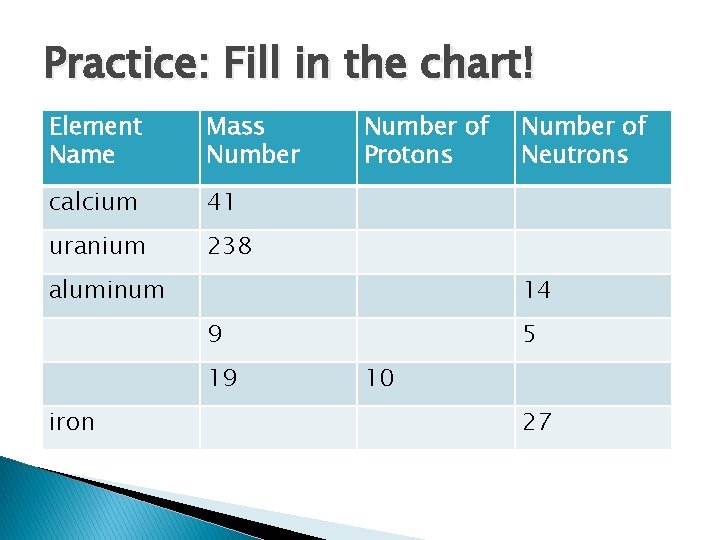
Practice: Fill in the chart! Element Name Mass Number calcium 41 uranium 238 Number of Protons aluminum 14 9 19 iron Number of Neutrons 5 10 27
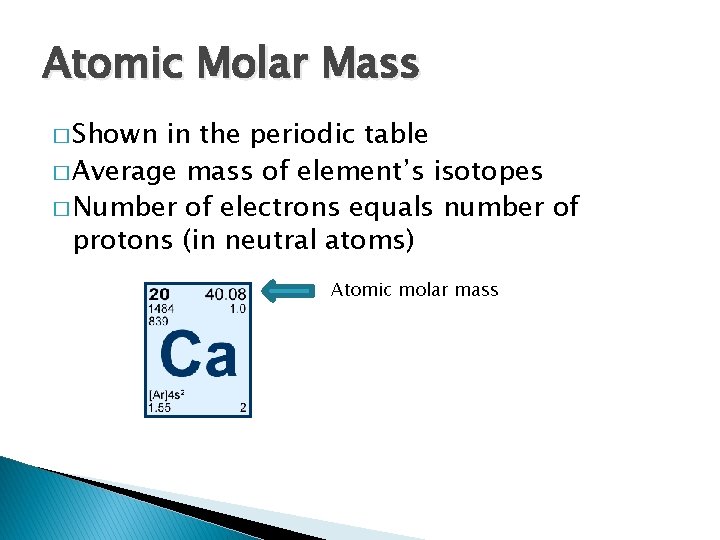
Atomic Molar Mass � Shown in the periodic table � Average mass of element’s isotopes � Number of electrons equals number of protons (in neutral atoms) Atomic molar mass

Energy Levels � Electrons occupy energy levels ◦ Region around nucleus either empty or contain electrons ◦ Increase energy when get further from the nucleus � Specific number of electrons/level ◦ First level- 2 electrons ◦ Next levels- 8 electrons � Energy level can be empty, partly or completely filled
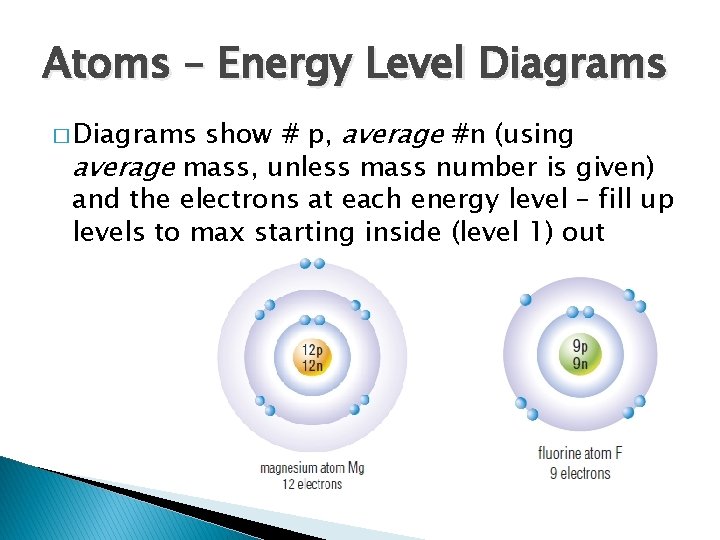
Atoms – Energy Level Diagrams show # p, average #n (using average mass, unless mass number is given) and the electrons at each energy level – fill up levels to max starting inside (level 1) out � Diagrams
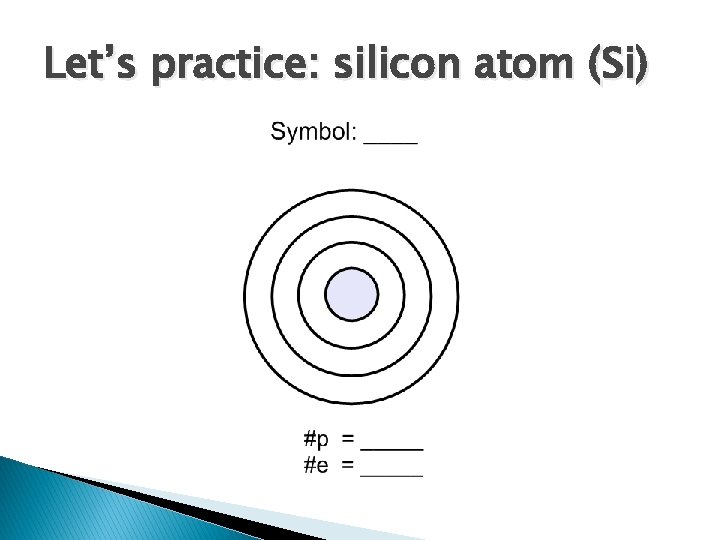
Let’s practice: silicon atom (Si)
The Periodic Table and Atomic Structure cont… Section 2. 1

Review � What are three subatomic particles in an atom? � What particles make up most of the mass? � How are electrons arranged? � How many electrons can be in the first energy level? Second? � What is an isotope? How can we find number of neutrons in an atom? � How do we know how many electrons we have?

Objectives � describe the process of ionization and formation of cations and anions � identify patterns in the periodic table for valence electrons and energy levels � relate ionization to the octet rule

Drawing Atoms � Drawings include: ◦ Number of protons ◦ Number of neutrons ◦ Electrons in correct energy levels (how many electrons can be in each level? ) � Ex. Magnesium atom � Oxygen � Calcium � Chlorine � Argon � Carbon-14

Valence Electrons � Electrons in the outermost energy level � Valence number- number of electrons an element can lose/gain to combine with other elements � How many valence electrons do each of the following elements have? ◦ ◦ ◦ Oxygen? Magnesium? Chlorine? Argon? Carbon-14?
Trends in the Periodic Table
The Octet Rule � For understanding how atoms bond � Atoms want to have eight electrons (filled) in their valence shell (which shell is this? ) � More stable when have full energy levels � What are these elements like with full energy levels? ◦ Noble gases! ◦ Gain/lose electrons to be like their closest noble gas ◦ Ex. Chlorine will gain one electron to be like argon ◦ Exception: hydrogen, lithium and beryllium. Why? Who do they want to be like?

What Now? � When we gain/lose electrons from an atom, what happens to its charge? ◦ Ex. Fluorine gains one electron to fill its outer octet. What is its electrical charge now? � Gaining/losing ionization electrons process called ◦ Results in positively charged or negatively charged ions
Formation of Ions � Elements electrons ◦ ◦ can lose or gain outermost (valence) Called ionization Makes ions (positively or negatively charged) Allows metals and non-metals to form compounds http: //www. youtube. com/watch? v=x. Tx_DWbo. EVs&f eature=related

Cations � Positively charged ions � Metal ion loses electrons � Where do the electrons go? ◦ To another atom � Why would the atom be positively charged if it loses electrons? � Ex Magnesium ◦ Loses 2 electrons ◦ What would the diagram look like before? After ionization? � Put charge as a superscript: ex. Mg 2+

Anions � Negatively charged ions � Non-metal gains electrons � Where do the electrons come from? ◦ From atoms that lose electrons (cations) � Why is it negatively charged? � Ex. Oxygen ◦ Gains two electrons ◦ What is the diagram before? After ionization? How would you write it as a symbol?

Naming Ions � Cations: ◦ Element name + ion ◦ Ex. sodium ion ◦ Ex. magnesium ion � Anions: ◦ First part of element name and change ending to “ide” + ion ◦ Ex. nitride ion ◦ Ex. Oxide ion ◦ Ex. Fluoride ion

Practice � For each of the following ions, draw with nucleus and electrons and give appropriate name or symbol: � Hydrogen ion � Cl� Nitride ion � Be 2+ � Sulfide ion
Ion trends in the Periodic table
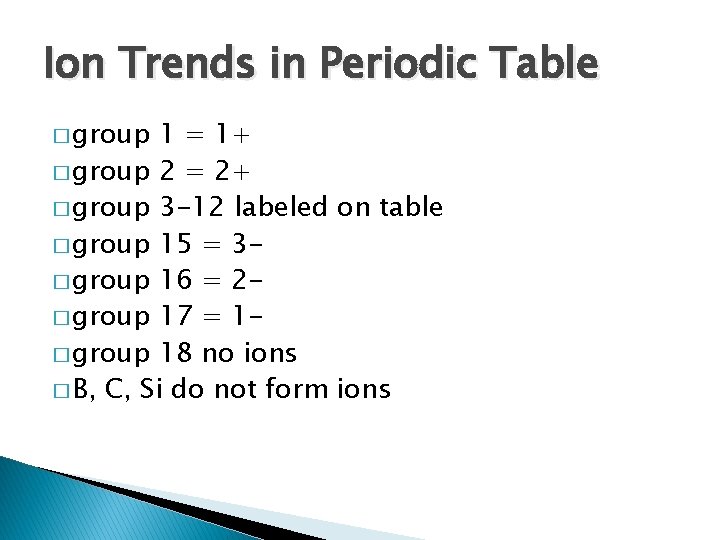
Ion Trends in Periodic Table � group 1 = 1+ � group 2 = 2+ � group 3 -12 labeled on table � group 15 = 3� group 16 = 2� group 17 = 1� group 18 no ions � B, C, Si do not form ions

Why form ions? � Why do atoms gain or lose electrons? ◦ To have full energy levels and be more stable like closest noble gas � Once ions are formed: ◦ Electrically charged ◦ What would happen when I bring a cation and an anion together? ◦ Form a bond! How we make compounds between metals and non-metals ◦ Need to form the ion first before a bond can be formed

Question � What is the difference between an atom and an ion? � How can I tell the difference with our diagrams? With the symbols?
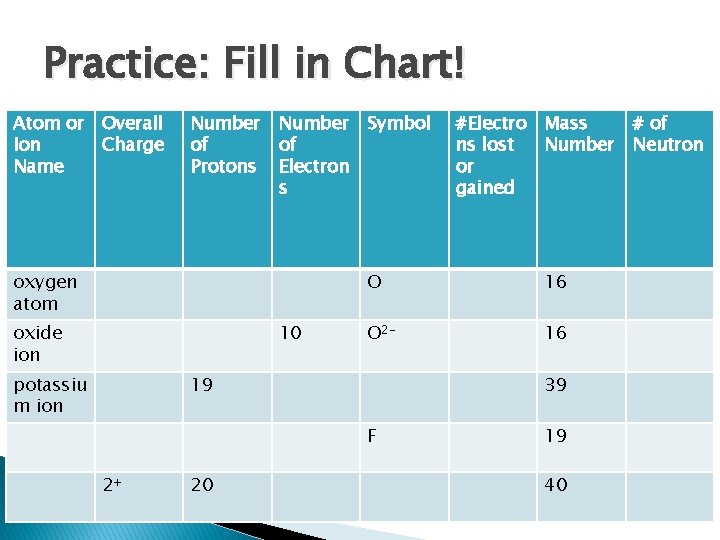
Practice: Fill in Chart! Atom or Overall Ion Charge Name Number of Protons Number of Electron s oxygen atom oxide ion 10 potassiu m ion Symbol O 16 O 2 - 16 19 39 F 2+ 20 #Electro Mass ns lost Number or gained 19 40 # of Neutron
Review Animation – Ionic Bonds � Ionic Bonds Gizmo to show ionic bonding and octet formation with charges http: //www. learnalberta. ca/Search. aspx? lang=en&search=gizmo+ionic+bon d&grade=&subject=

Homework � Read pg. 34 -38 � Pg. 39 # 4 -12 � Workbook p. 7 -9
- Slides: 45