The Periodic Table A Chemists Most Important Tool

The Periodic Table A Chemist’s Most Important Tool
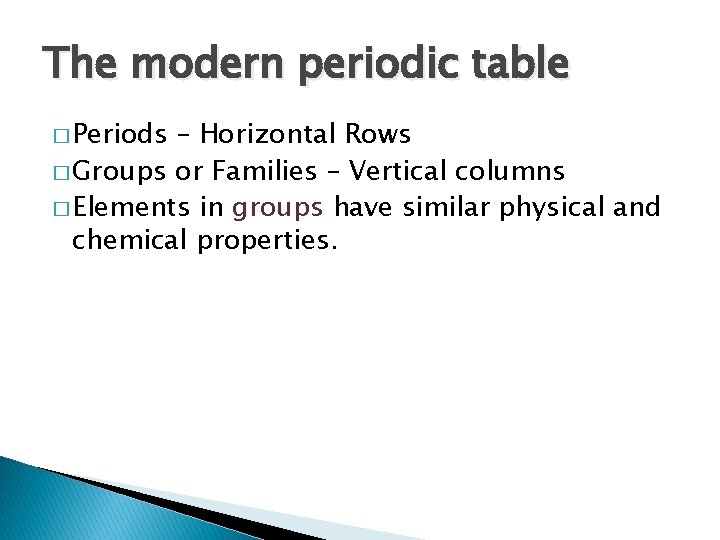
The modern periodic table � Periods – Horizontal Rows � Groups or Families – Vertical columns � Elements in groups have similar physical and chemical properties.
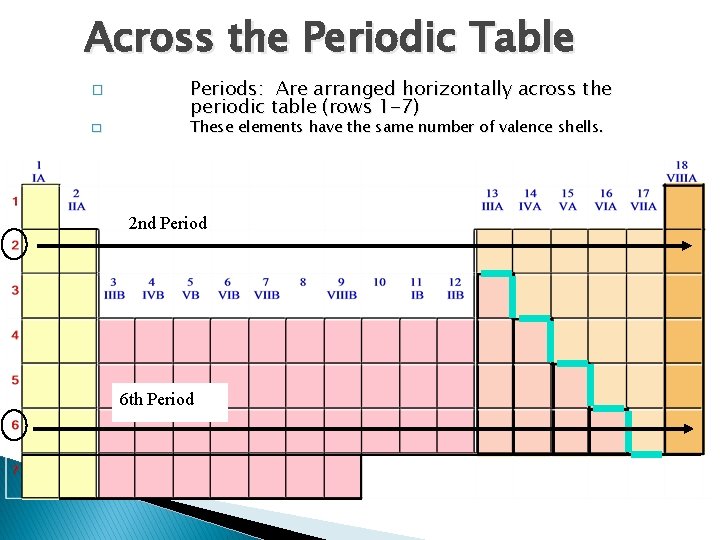
Across the Periodic Table � � Periods: Are arranged horizontally across the periodic table (rows 1 -7) These elements have the same number of valence shells. 2 nd Period 6 th Period
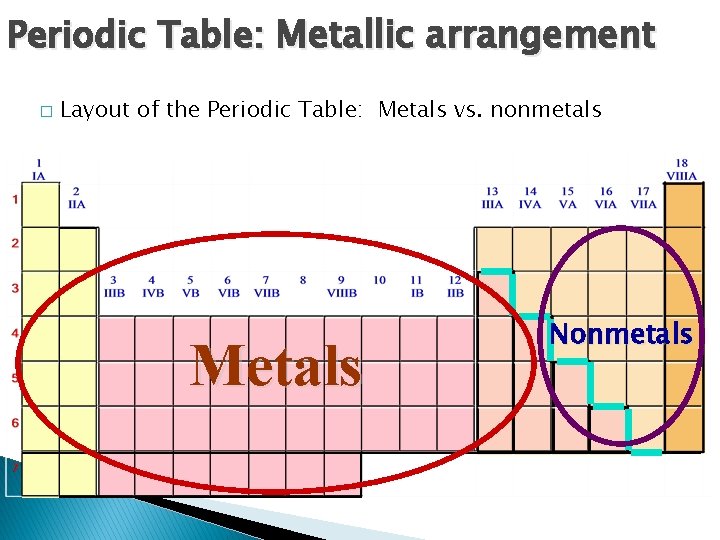
Periodic Table: Metallic arrangement � Layout of the Periodic Table: Metals vs. nonmetals Metals Nonmetals
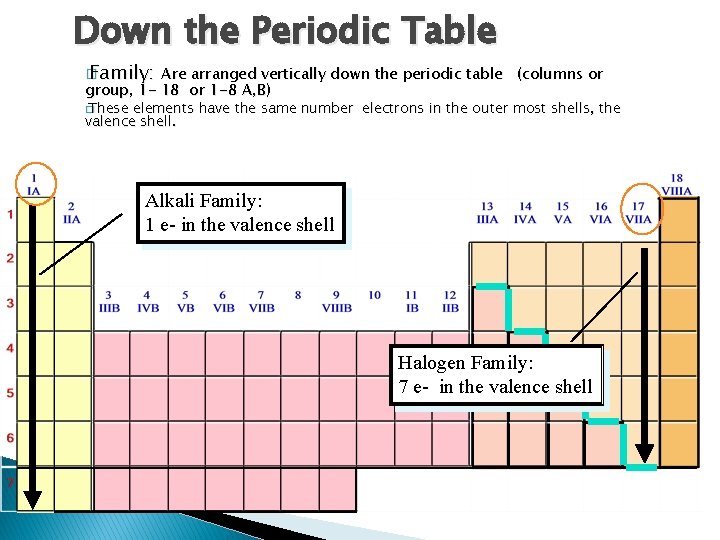
Down the Periodic Table � Family: Are arranged vertically down the periodic table group, 1 - 18 or 1 -8 A, B) (columns or � These elements have the same number electrons in the outer most shells, the valence shell. Alkali Family: 1 e- in the valence shell Halogen Family: 7 e- in the valence shell
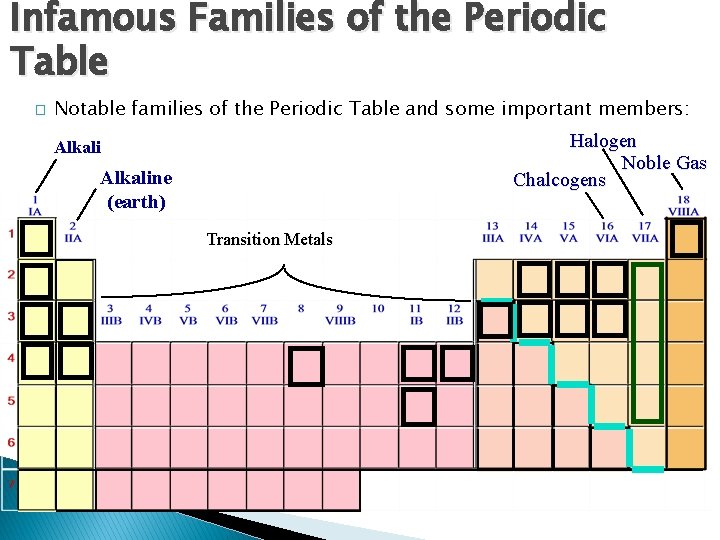
Infamous Families of the Periodic Table � Notable families of the Periodic Table and some important members: Halogen Noble Gas Chalcogens Alkaline (earth) Transition Metals
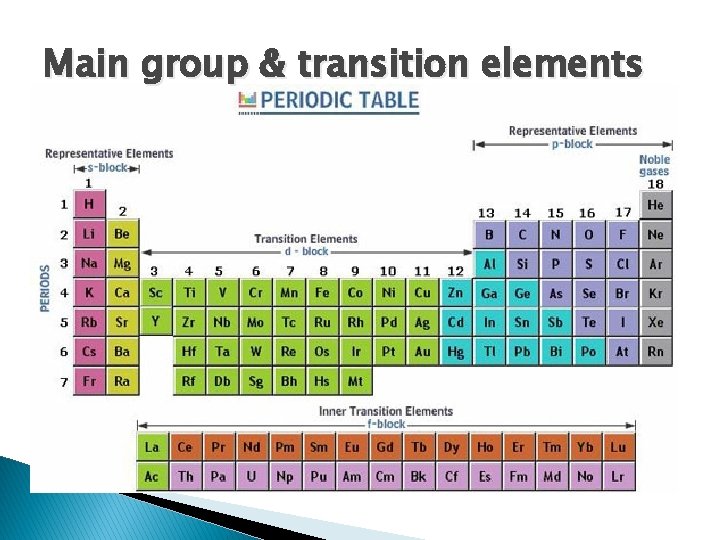
Main group & transition elements
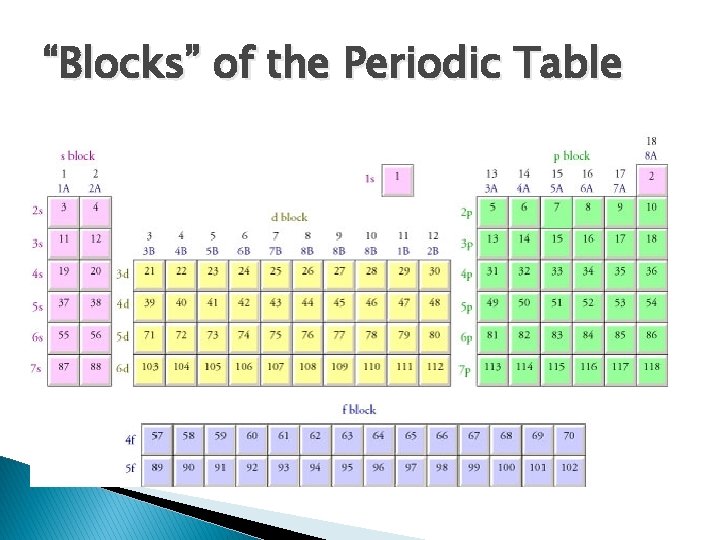
“Blocks” of the Periodic Table
The Development of the Periodic Table � During the nineteenth century, chemists began to categorize the elements according to similarities in their physical and chemical properties. The end result of these studies was our modern periodic table.
Early Classification � Dobereiner (1829) ◦ Model of triads �Groups of 3 ie: Ca, Sr, Ba OR Cl, Br, I � Newlands (1863) ◦ Law of Octaves �Worked well for first 20 elements

The First Periodic Table � Dmitri (1869) Mendeleev ◦ Arranged elements by increasing atomic mass ◦ Noticed a regular pattern in chemical and physical properties ◦ Left blank spaces – Predicted the existence of elements not yet discovered based on properties
Support for Mendeleev’s Table • He was so confident in his table that he used it to predict the physical properties of three elements that were yet unknown. • After discovery of Sc, Ga, & Ge his predictions were shown to be correct, lending support to his table.
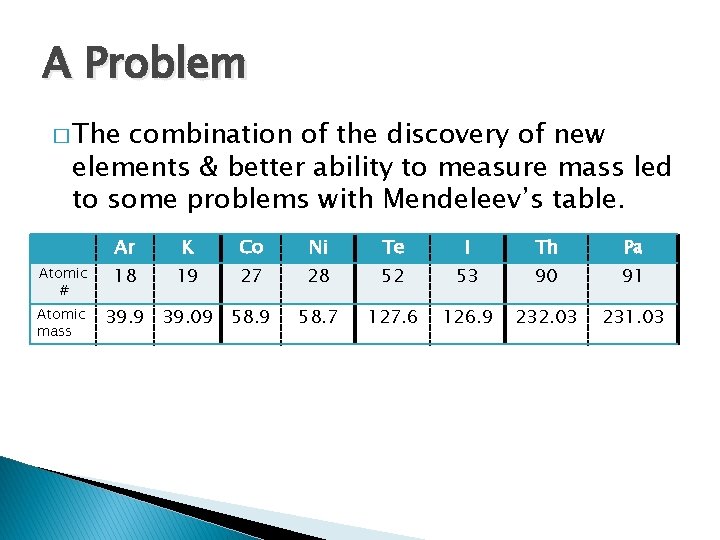
A Problem � The combination of the discovery of new elements & better ability to measure mass led to some problems with Mendeleev’s table. Atomic # Atomic mass Ar K Co Ni Te I Th Pa 18 19 27 28 52 53 90 91 58. 9 58. 7 127. 6 126. 9 232. 03 231. 03 39. 9 39. 09

A new arrangment � In 1913 Henry Moseley was able to determine the nuclear charges of the elements � He used this information to rearrange the elements in order of atomic number. � His research was halted when the British government sent him to serve as a foot soldier in WWI. He was killed in the fighting in Gallipoli by a sniper’s bullet, at the age of 28. Because of this loss, the British government later restricted its scientists to noncombatant duties during WWII.
A “final” adjustment � After co-discovering 10 new elements, in 1944 Glenn Seaborg moved 14 elements from the body of the periodic table to the Actinide Series. � He is the only person to have an element named after him while he was still alive.

Periodic Law � When elements are arranged in order of increasing atomic number, there is a regular pattern in their chemical and physical properties.
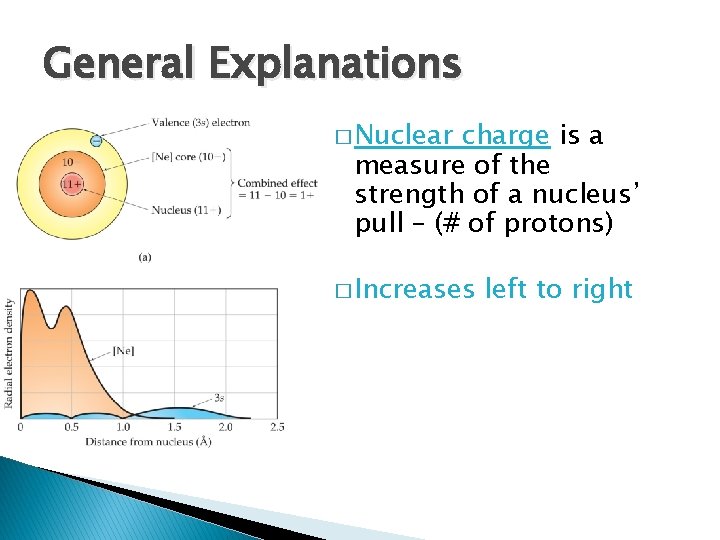
General Explanations � Nuclear charge is a measure of the strength of a nucleus’ pull – (# of protons) � Increases left to right
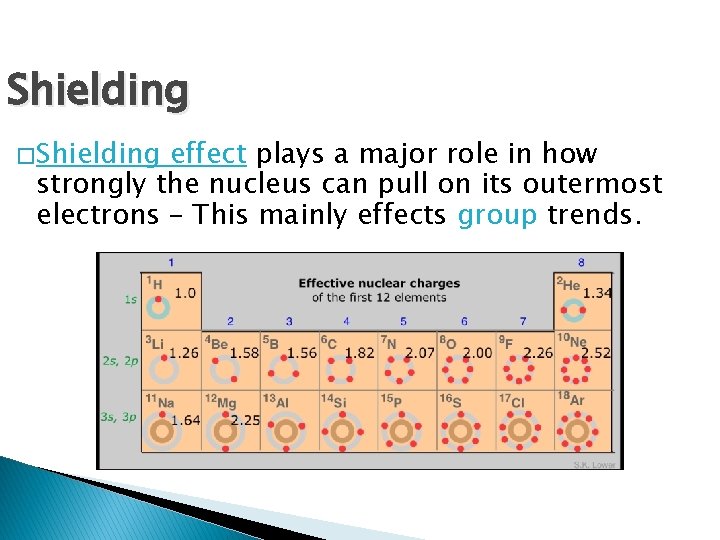
Shielding � Shielding effect plays a major role in how strongly the nucleus can pull on its outermost electrons – This mainly effects group trends.
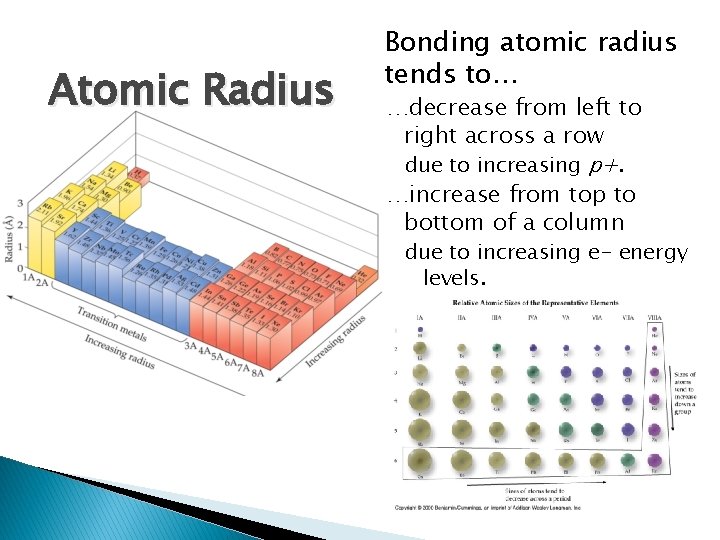
Atomic Radius Bonding atomic radius tends to… …decrease from left to right across a row due to increasing p+. …increase from top to bottom of a column due to increasing e- energy levels.
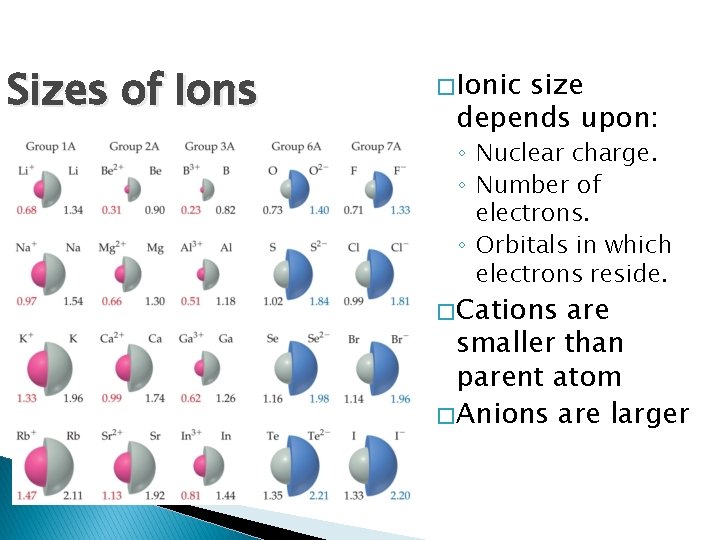
Sizes of Ions � Ionic size depends upon: ◦ Nuclear charge. ◦ Number of electrons. ◦ Orbitals in which electrons reside. � Cations are smaller than parent atom � Anions are larger
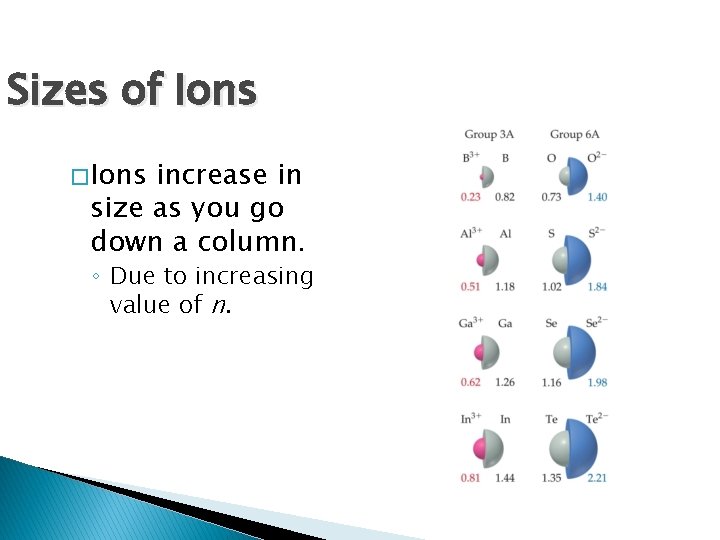
Sizes of Ions � Ions increase in size as you go down a column. ◦ Due to increasing value of n.
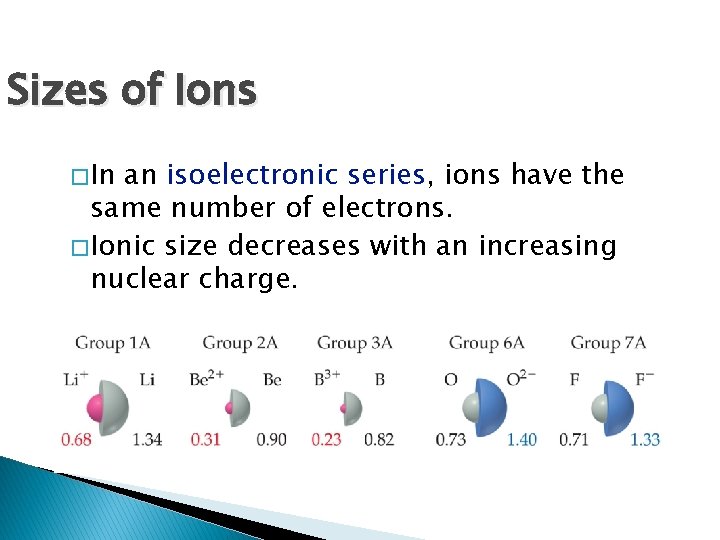
Sizes of Ions � In an isoelectronic series, ions have the same number of electrons. � Ionic size decreases with an increasing nuclear charge.

Ionization Energy � Amount of energy required to remove an electron from the ground state of a gaseous atom or ion. ◦ First ionization energy is that energy required to remove first electron. ◦ Second ionization energy is that energy required to remove second electron, etc.
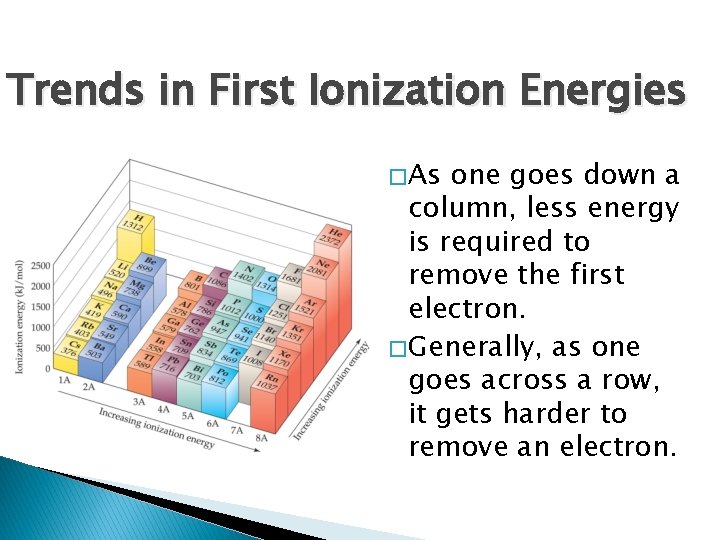
Trends in First Ionization Energies � As one goes down a column, less energy is required to remove the first electron. � Generally, as one goes across a row, it gets harder to remove an electron.
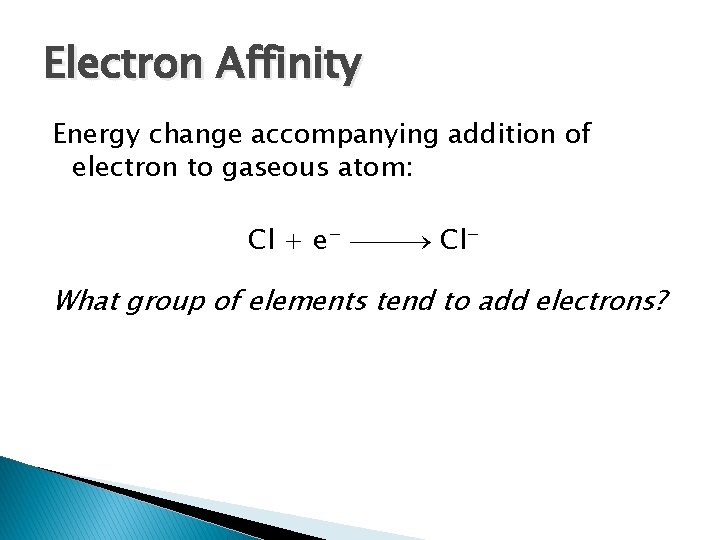
Electron Affinity Energy change accompanying addition of electron to gaseous atom: Cl + e− Cl− What group of elements tend to add electrons?
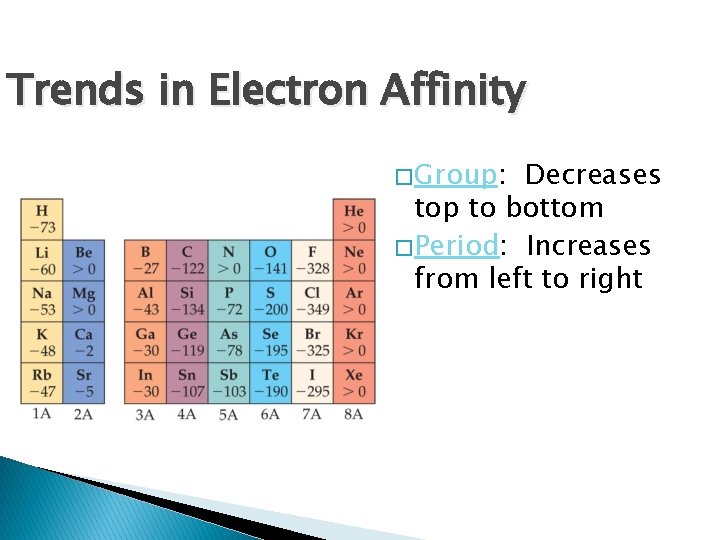
Trends in Electron Affinity � Group: Decreases top to bottom � Period: Increases from left to right
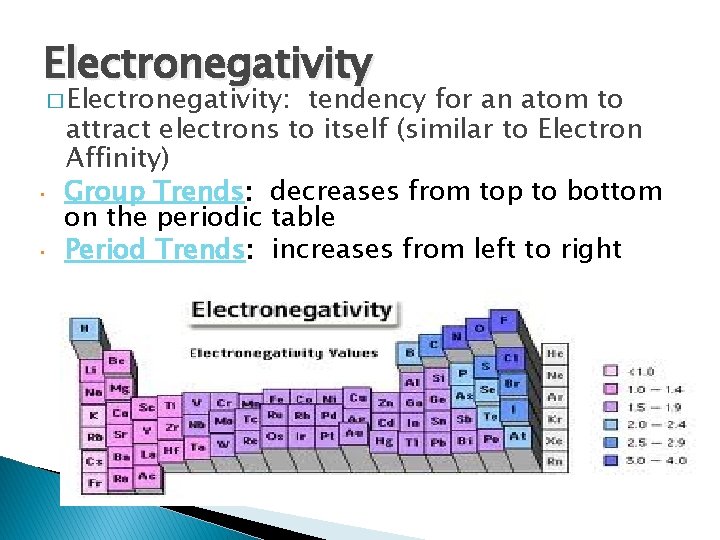
Electronegativity � Electronegativity: • • tendency for an atom to attract electrons to itself (similar to Electron Affinity) Group Trends: decreases from top to bottom on the periodic table Period Trends: increases from left to right
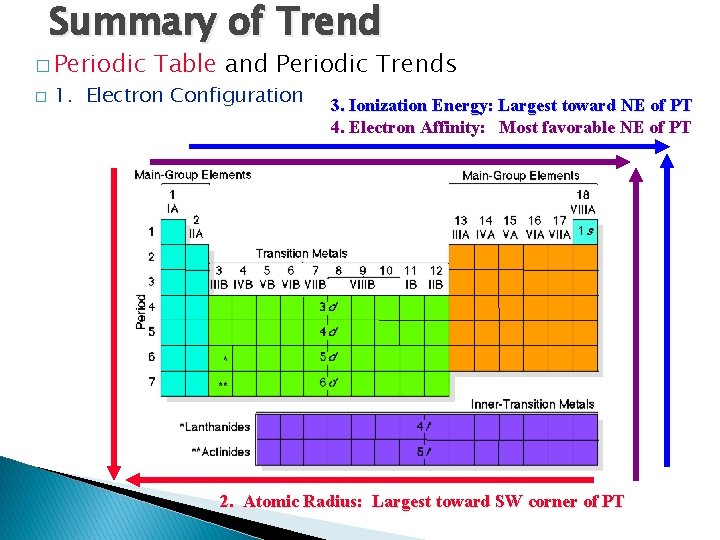
Summary of Trend � Periodic � Table and Periodic Trends 1. Electron Configuration 3. Ionization Energy: Largest toward NE of PT 4. Electron Affinity: Most favorable NE of PT 2. Atomic Radius: Largest toward SW corner of PT
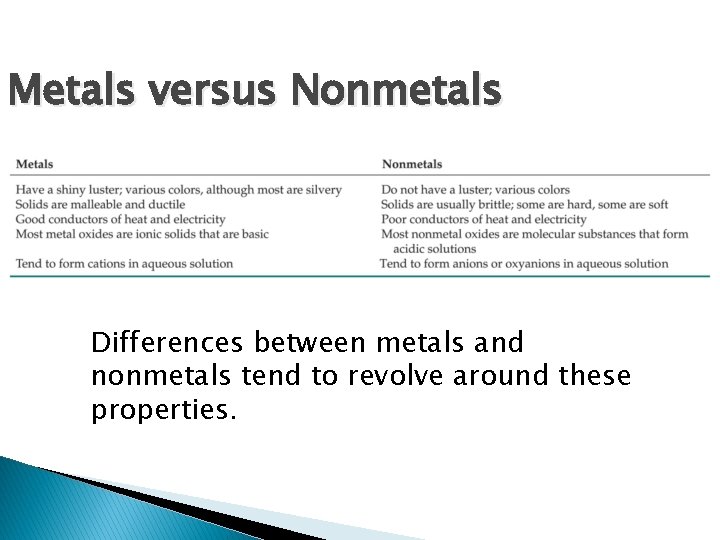
Metals versus Nonmetals Differences between metals and nonmetals tend to revolve around these properties.
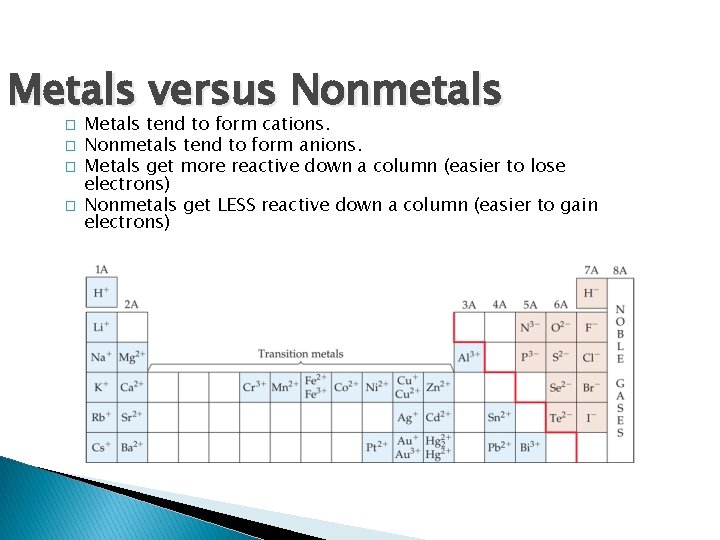
Metals versus Nonmetals � � Metals tend to form cations. Nonmetals tend to form anions. Metals get more reactive down a column (easier to lose electrons) Nonmetals get LESS reactive down a column (easier to gain electrons)
- Slides: 30