The Periodic Table 6 3 Periodic Trends Atomic

The Periodic Table 6. 3 Periodic Trends
Atomic Size • Atoms are very small so we can’t measure their size directly. • When atoms of the same element are joined together, molecules are formed. • The atomic radius can be determined by taking half of the distance between two atoms. • Measured in picometers (1 trillion pm in a m)
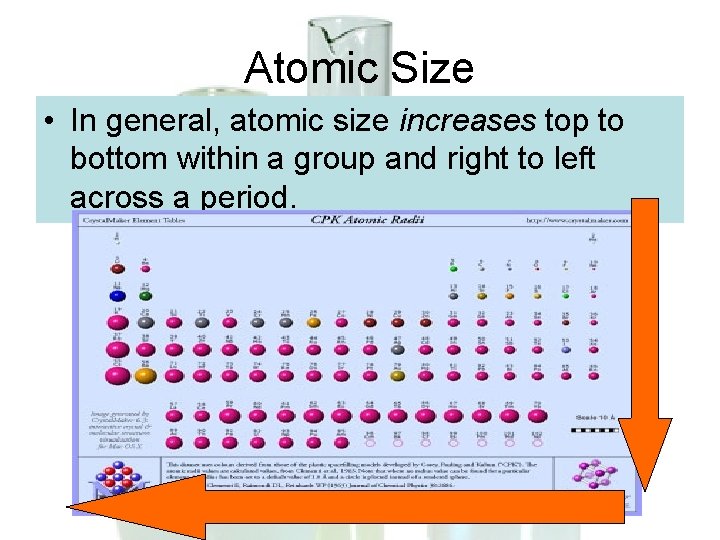
Atomic Size • In general, atomic size increases top to bottom within a group and right to left across a period.

Ions • Ion: atom with a positive/negative charge. • Happens when ELECTRONS are transferred between atoms. • Positive charge = CATION (less electrons) • Negative charge = ANION (more electrons)
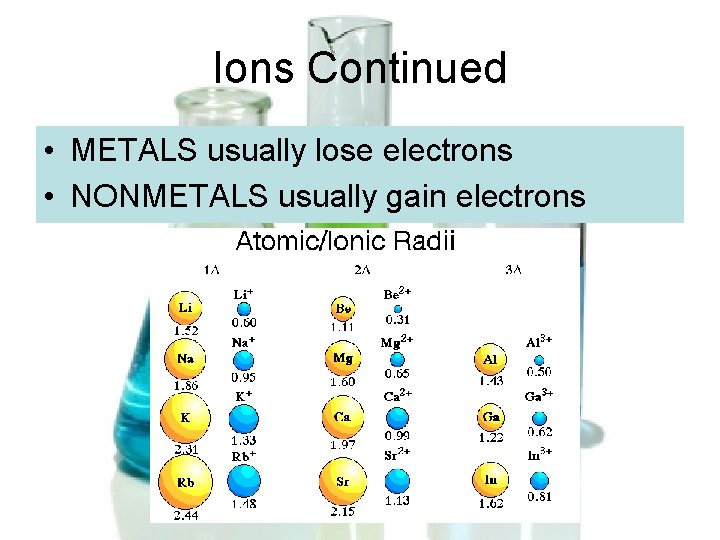
Ions Continued • METALS usually lose electrons • NONMETALS usually gain electrons
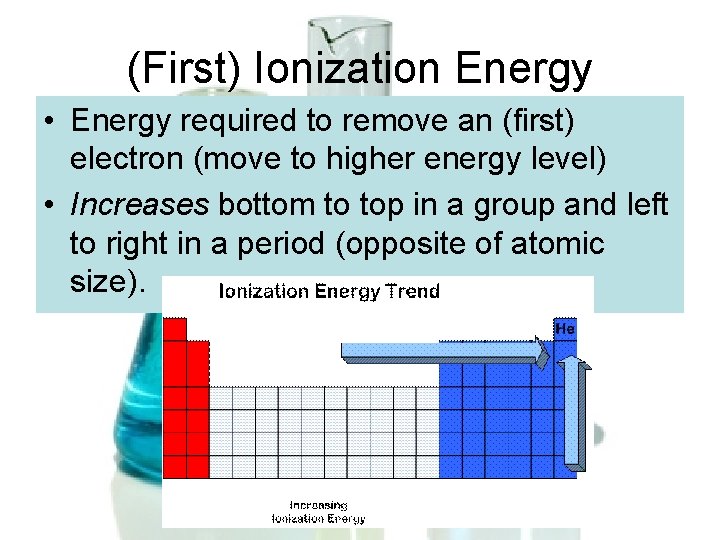
(First) Ionization Energy • Energy required to remove an (first) electron (move to higher energy level) • Increases bottom to top in a group and left to right in a period (opposite of atomic size).
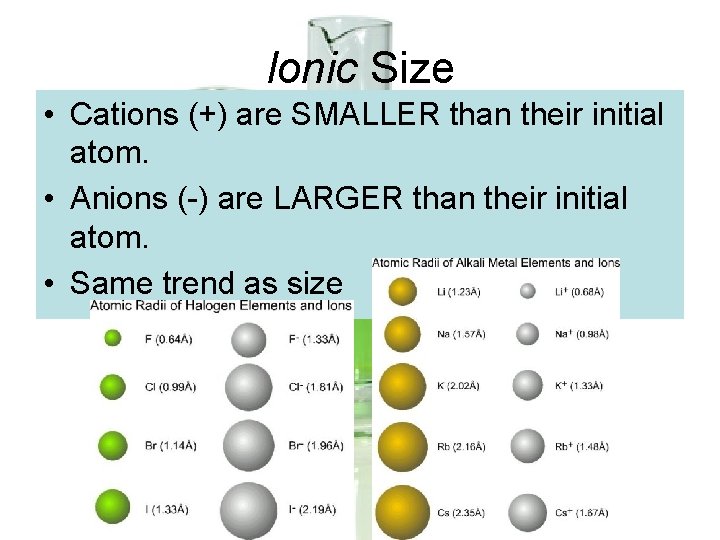
Ionic Size • Cations (+) are SMALLER than their initial atom. • Anions (-) are LARGER than their initial atom. • Same trend as size
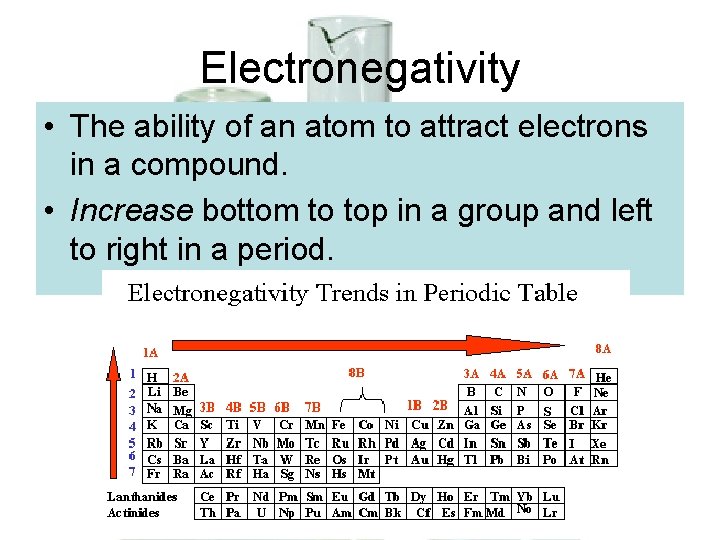
Electronegativity • The ability of an atom to attract electrons in a compound. • Increase bottom to top in a group and left to right in a period.

Electronegativity Continued • LEAST electronegative = cesium (tends to LOSE electrons in reactions) • MOST electronegative = fluorine (tends to GAIN electrons in reactions)
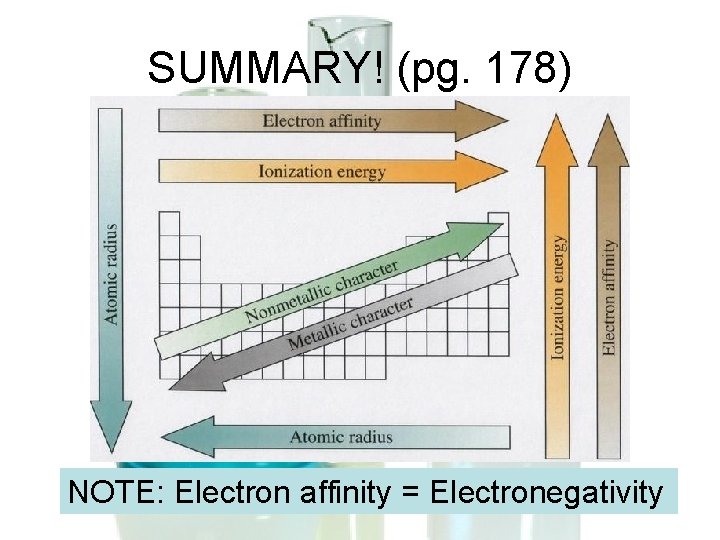
SUMMARY! (pg. 178) NOTE: Electron affinity = Electronegativity
- Slides: 11