The Periodic Table 3 1 The Periodic Table

The Periodic Table!!!!

3. 1 The Periodic Table • History • Organization • Electron arrangement & valence energy level

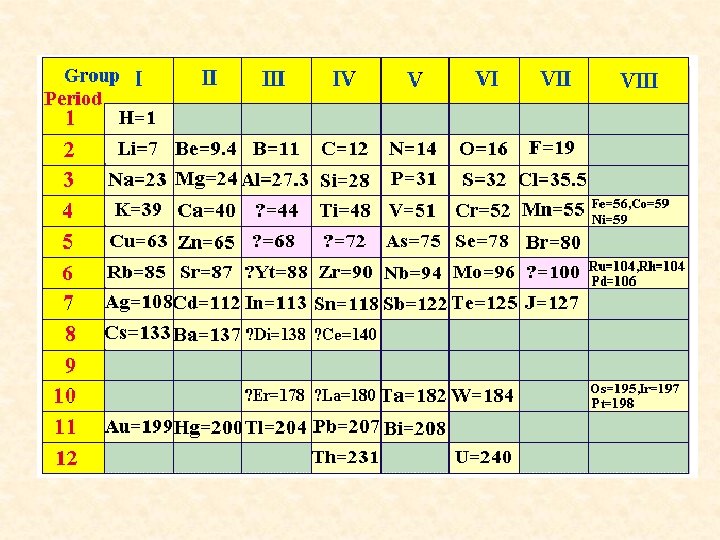
Dmitri Mendeleev • 1834 -1907; sequenced known elements in order of increasing atomic mass • Elements with similar properties in same column. • Called Periodic Table because it highlighted the repeated (periodic) patterns of properties.

Periodic Law • Chemical & physical properties of the elements repeat in a regular, periodic pattern when arranged by atomic number • This is the basis for the modern periodic table – arranged by atomic number.

The Modern Periodic Table • Using the information in your data booklet, complete the periodic table worksheet. • Be sure to include a legend on your worksheet.
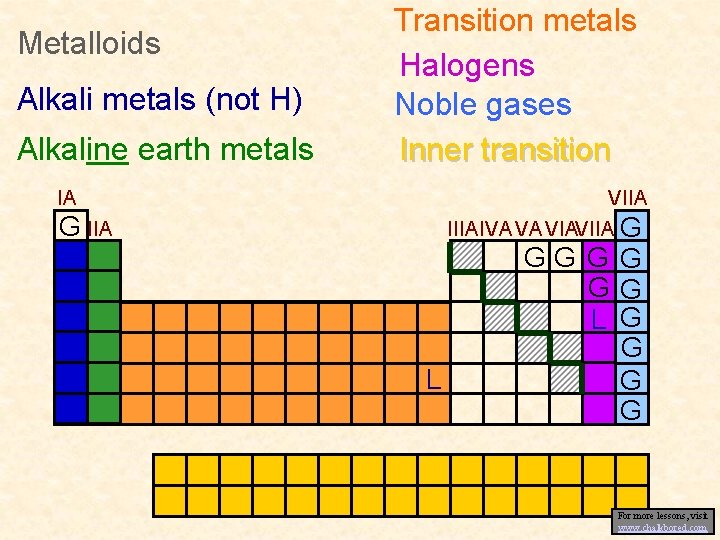
Metalloids Alkali metals (not H) Alkaline earth metals Transition metals Halogens Noble gases Inner transition IA VIIA IIIAIVA VA VIAVIIA G G IIA L GG GG GG L G G For more lessons, visit www. chalkbored. com

• Group Names: – Two systems: Old & New • Old: Roman numerals with either A or B • New: Integers 1 -18 from left to right • Group VA = Group 15 • Alkali Metals: – Group 1 – Soft, light, reactive metals • Alkaline Earth Metals: – Group 2 – Harder, denser, and stronger than alkali metals

• Transition Metals: – Group 3 -12 – Form positive ions, reactive, make coloured compounds • Metalloids: – On staircase between metals and non-metals – Exhibits properties of both

• Halogens: – Group 17; Reactive non-metals – Electron deficient and readily share electrons with other elements • Noble Gases: – Group 18 – Do not react chemically with other materials and cannot be absorbed – Valence shell full
The Periodic Table & Electrons • Periodic trends are linked to the way electrons fill energy levels. • Remember that for each proton there is one electron in an atom, therefore as the atomic number increases, so does the number of electrons. • To understand trends, we use the Bohr. Rutherford Model of the atom.
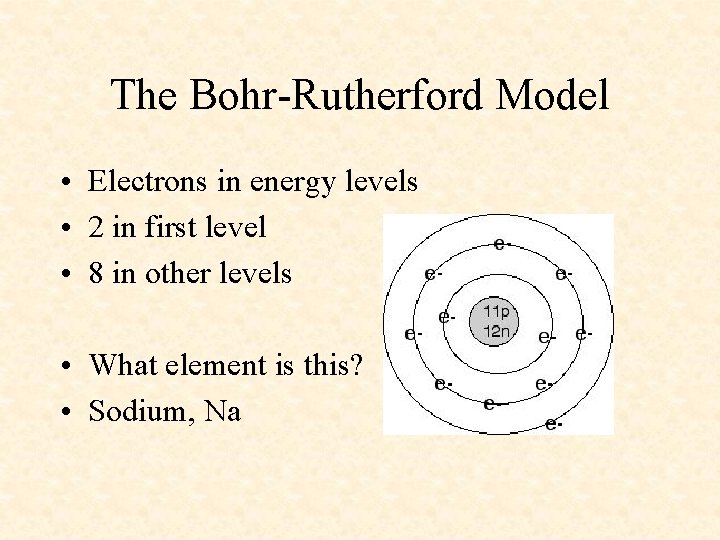
The Bohr-Rutherford Model • Electrons in energy levels • 2 in first level • 8 in other levels • What element is this? • Sodium, Na
• Group Related Pattern: – All the elements in a group have the same number of valence electrons. – The old number system – The roman numeral is the same as the number of valence electrons. – The new system – The last digit of the number is the number of valence electrons. • Period-Related Pattern ØPeriod Number = Number of Energy Levels

3. 2 Physical Properties • • Ionization energy Electronegativity Atomic Radii Ionic Radii • Across period 3 & down group 1 & 17

More Trends – Worksheet • Ionization Energy: The energy required to remove one valence electron from an atom. • Electronegativity: How strongly an atom attracts the electrons in a covalent bond • Atomic Radius: The distance from the nucleus to the valence electron shell of an atom • Ionic Radius: The distance from the nucleus to the valence electron shell of an ion
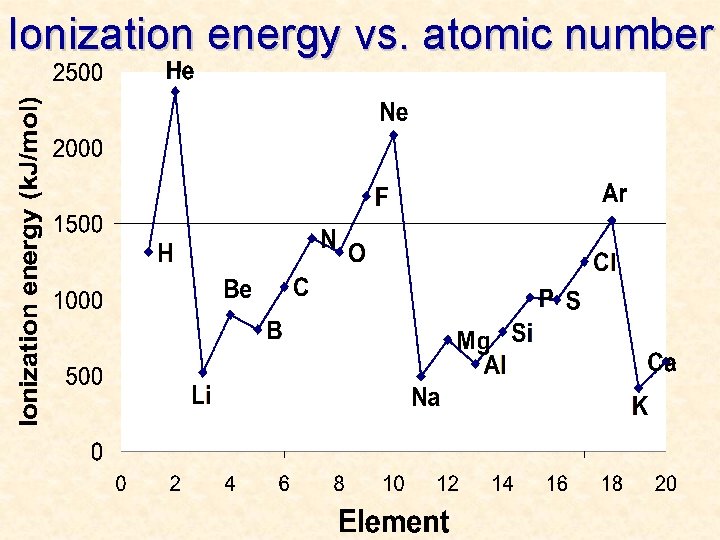
Ionization energy vs. atomic number
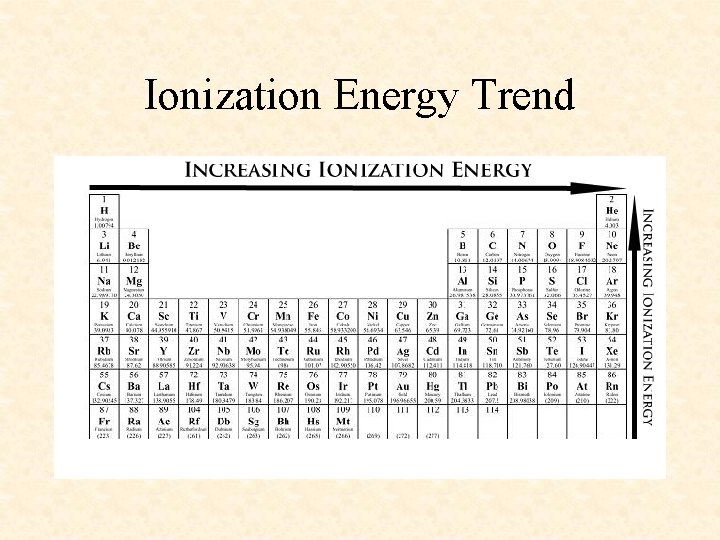
Ionization Energy Trend
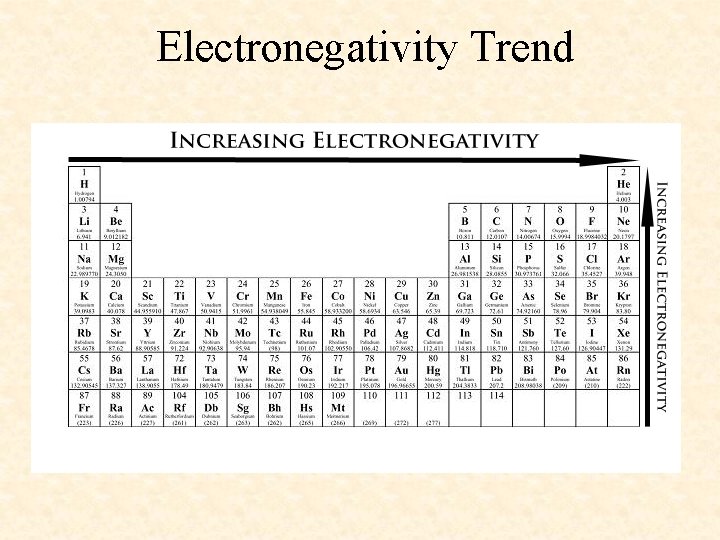
Electronegativity Trend
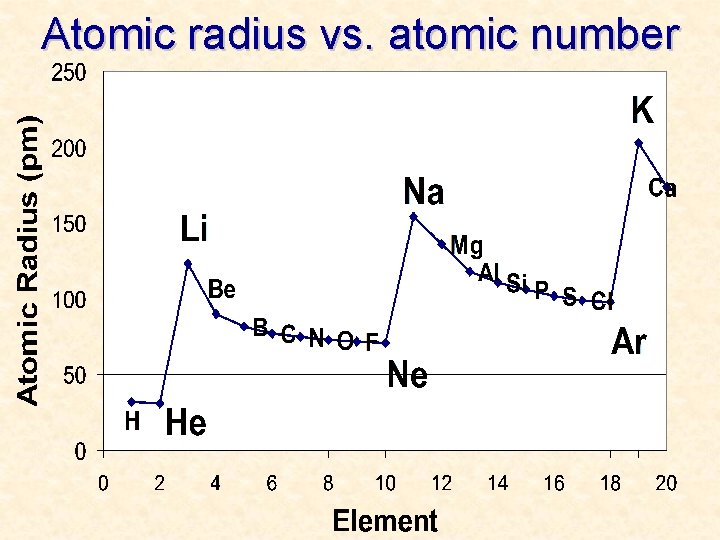
Atomic radius vs. atomic number
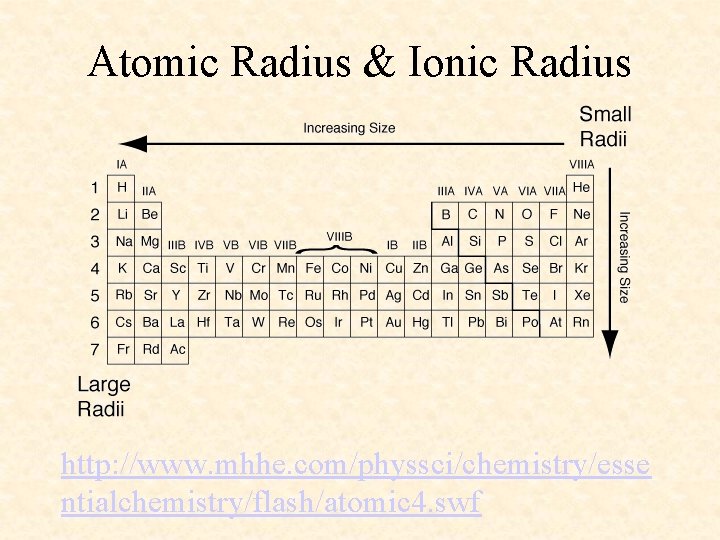
Atomic Radius & Ionic Radius http: //www. mhhe. com/physsci/chemistry/esse ntialchemistry/flash/atomic 4. swf
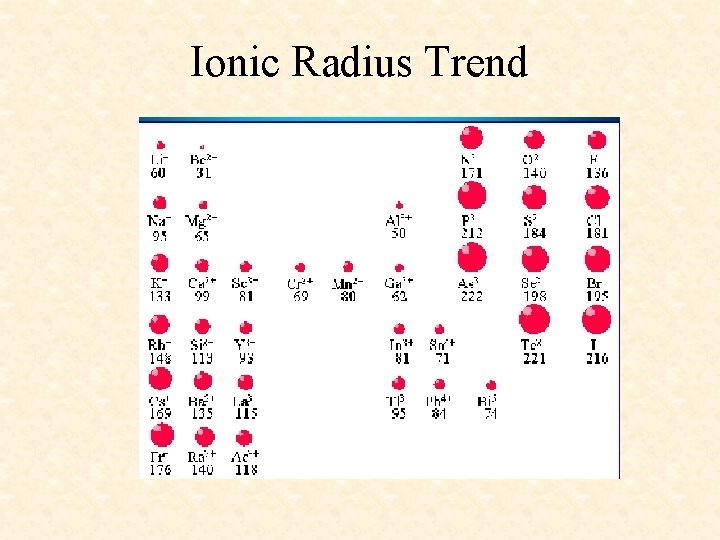
Ionic Radius Trend

3. 3 Chemical Properties • Group Properties: – Alkali metals with water – Alkali metals with halogens – Halogens with water – Halogens with halide ions • Oxides of period 3 properties – Ionic vs. covalent bonding nature – Basic vs. acidic nature – Industrial processes

Alkali metals with H 2 O • M + H 2 O MOH + H 2 • Trend: Very reactive; increasing down group • Observations: – Highly exothermic – heat & light – Fizzing of hydrogen gas formed – Video
Alkali Metals with Halogens • M + X 2 MX • React in 1: 1 ratio

Halogens with H 2 O • Reactivity decreases down group because electronegativity and oxidizing power decreases down group; electrons farther from nucleus and are shielded • Halogens General Video
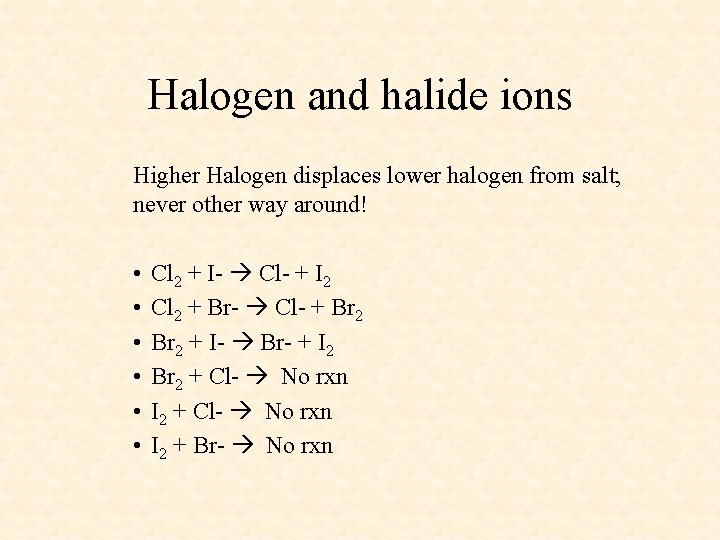
Halogen and halide ions Higher Halogen displaces lower halogen from salt; never other way around! • • • Cl 2 + I- Cl- + I 2 Cl 2 + Br- Cl- + Br 2 + I- Br- + I 2 Br 2 + Cl- No rxn I 2 + Br- No rxn

Halogen and metal • Make salts with halide ion • Salts are usually white in colour and soluble in water creating colourless solutions • Common insoluble halides: silver and lead

Things to Know: • Ionic compound– attraction of ions creates bond • Covalent compound – shared electrons create bond • Polar Covalent – a covalent bond where electrons are not shared equally creating positive and negative “ends” on the bond • Acidic – having a p. H of less than 7; H+ • Basic – having a p. H of more than 7; OH

Oxides: • Compounds containing at least one oxygen atom • Bonded with metal: ionic compound & basic • Bonded with non-metal: polar covalent compound; polarity decreasing across period & acidic (increasing across period) • **aluminum oxide is amphoteric – reacts with acid and base**
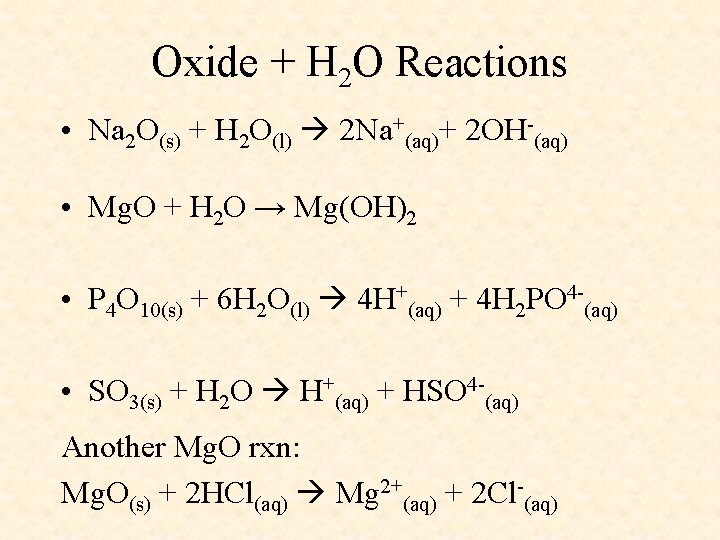
Oxide + H 2 O Reactions • Na 2 O(s) + H 2 O(l) 2 Na+(aq)+ 2 OH-(aq) • Mg. O + H 2 O → Mg(OH)2 • P 4 O 10(s) + 6 H 2 O(l) 4 H+(aq) + 4 H 2 PO 4 -(aq) • SO 3(s) + H 2 O H+(aq) + HSO 4 -(aq) Another Mg. O rxn: Mg. O(s) + 2 HCl(aq) Mg 2+(aq) + 2 Cl-(aq)
- Slides: 30