The Periodic Table 1 Beginning of the Periodic

The Periodic Table 1

Beginning of the Periodic Table ØDmitri Mendeleev ØDiscovered pattern of the elements Ø Arranged mass by increasing atomic ØPeriodic-occurs intervals in regular 2

Henry Moseley u Rearranged elements by increasing atomic number u Periodic Law-chemical and physical properties of elements change periodically with the atomic numbers of the elements 3

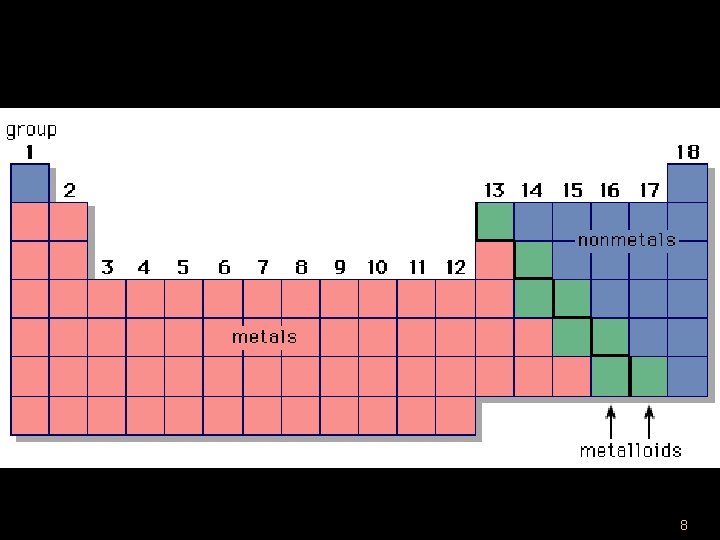
The Periodic Table u 115 elements u 3 classes of elements – Metals – Nonmetals – Metalloids u Amount of electrons in the outermost level helps determine class 4

Metals u Key Properties – Shiny – Malleable (bendable) – Ductile – Good conductors of electricity and thermal energy – Solid at room temperature (except Hg) u Found to the left of the zig-zag 5

Nonmetals u Key Properties (opposite of metals) – Not malleable or ductile – Not shiny – Poor conductors of thermal energy and electricity u Found to the right of the 6

Metalloids u Semiconductors u Have some properties of metals and some of nonmetals u Border the zig-zag 7
8

Reading the Periodic Table u Each square has: – Element’s Name – Chemical Symbol – Atomic Number – Atomic Mass 9
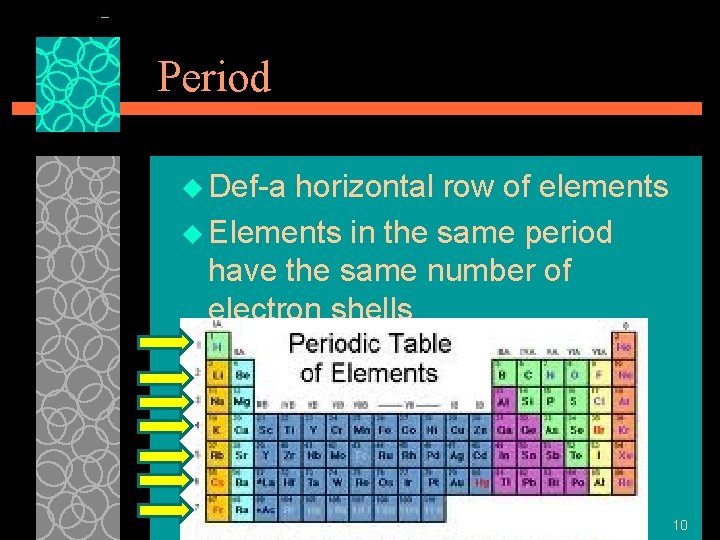
Period u Def-a horizontal row of elements u Elements in the same period have the same number of electron shells 10
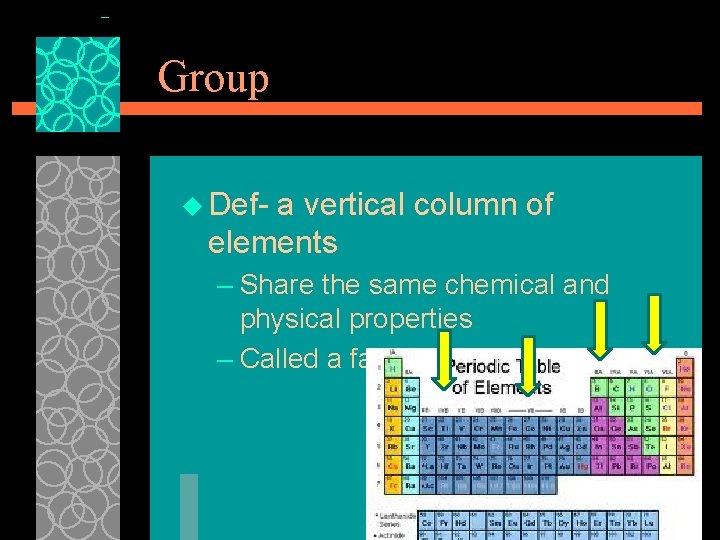
Group u Def- a vertical column of elements – Share the same chemical and physical properties – Called a family 11

Group 1: Alkali Metals u Most reactive metals – So reactive they are only found as compounds u Properties: – Soft – Color (silver) – Shiny – Low density u Ex: Na. Cl – Sodium Chloride 12

Group 2: Alkaline-Earth Metals u Less reactive than Group 1 u Properties – Color (silver) – Higher density than alkali metals u Mixed with other elements to make things – Airplanes (Mg) or cement and chalk (Ca) 13

Groups 3 -12: Transition Metals u Properties: – Shiny – Good conductors of thermal energy and electricity – Higher density and melting point than Groups 1 &2 – Less reactive than Groups 1 &2 14

Lanthanides and Actinides u Two rows on the bottom the periodic table u Part of Transition metals group u Lanthanides Top row u Actinides Bottom row 15

Group 13: Boron Group u Properties: – Solid at room temperature – Reactive u Ex: Aluminum – Most common and most abundant 16

Group 14: Carbon Group u Properties: – Solid at room temperature u Carbon makes up proteins, fats, and carbohydrates t Essential for life 17

Group 15: Nitrogen group u. Nitrogen makes up 80% of the air we breathe u. Solid at room temperature 18

Group 16: Oxygen Group u Oxygen makes up 20% of the air we breathe u All elements in this group, except for Oxygen, are solid at room temperature 19

Group 17: Halogens u Properties: – Poor conductors of electricity – Violent reactions w/ alkali metals u Combine with most elements to form salts u Chemical properties are similar but physical properties very 20

Group 18: Noble Gases u Unreactive gases u Ex: Helium, Neon – Neon Lights 21

Hydrogen u In a group of its own u Properties: – Colorless – Odorless – Gas (at room temperature) – Low density – Explosive reaction with Oxygen u Most abundant element in the universe 22
- Slides: 22