The Periodic Law Modern Chemistry 2009 Holt Rinehart












- Slides: 22

The Periodic Law Modern Chemistry © 2009 Holt, Rinehart, & Winston Chapter 5, pp 133 - 165

Periodic Law • Chemical & physical properties regularly repeat when elements are listed according to their atomic numbers § Atomic number is equal to # of protons § # of protons = # of electrons § # of valence electrons = Group # for s block = (Group # - 10) for p block o “Main-group elements” are those in the s & p blocks o Properties are determined mostly by valence electrons

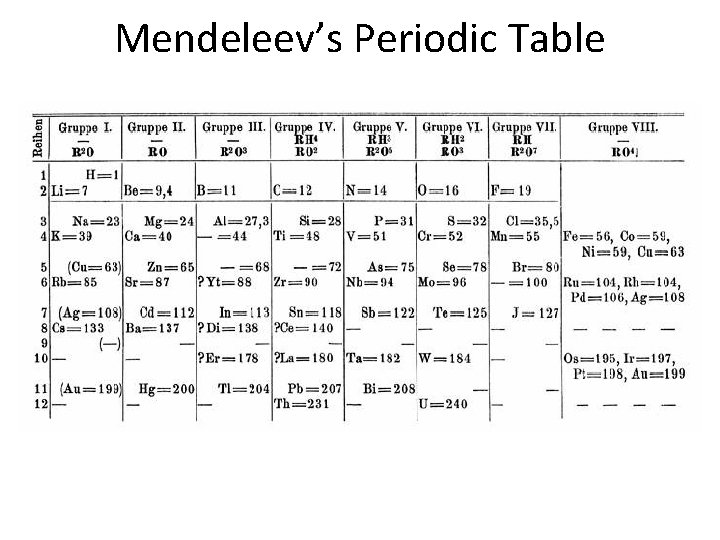
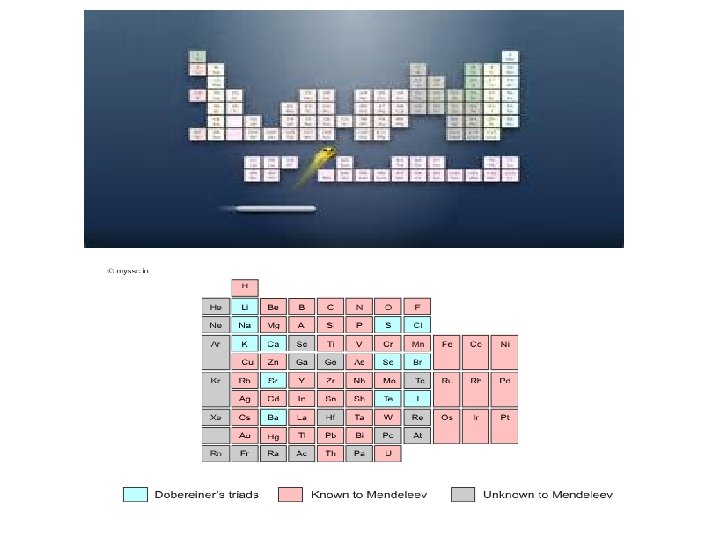
Contributors • Stanislao Cannizzaro – Reliable method of measuring atomic mass • Dmitri Mendeleev – Group elements of similar properties together – Arrange groups according to their atomic masses • Henry Moseley – Rearrange elements according to atomic numbers – Maintain groupings according to properties

Mendeleev’s Periodic Table


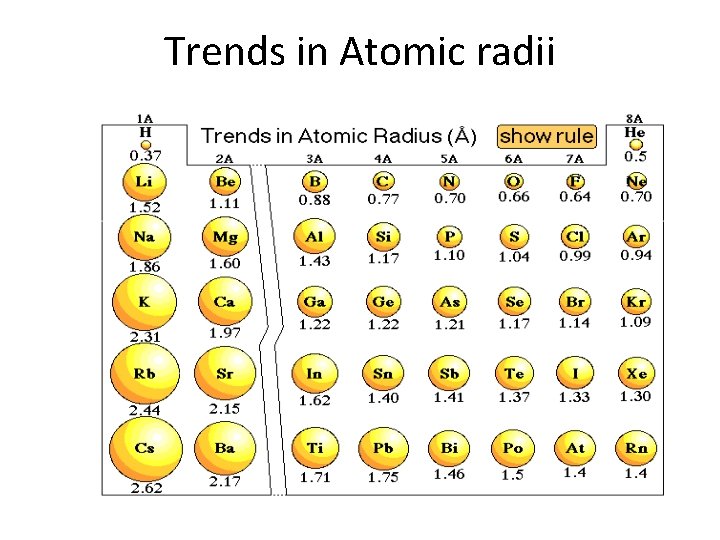
Periodic Properties’ Trends • Atomic & ionic radii: as go left & down – Across the period, nuclear charge increases. • As the positive charge increases, electrons are pulled in more tightly, thereby the radius. – Down the group, the # of energy levels increase. • Cations have smaller ionic radii than the atom. – When the valence electron shell is lost, the ion is smaller • Anions have larger ionic radii than the atom. – Extra electrons do not compact as readily, mostly because of their electrostatic repulsion for each other.

Trends in Atomic radii


Periodic Properties’ Trends • Ionization energy = energy required to remove electron from neutral atom § as you go right across the period o As nuclear charge increases, electrons are held more tightly. § as you go down the group o As energy levels are added, electrons are held more loosely. o Additional layers of electrons shielding of protons and further repel valence electrons.


Periodic Properties’ Trends • Electron affinity = energy change accompanying acquisition of electrons by neutral atoms. § Most atoms release energy when get electron. o Released energy is noted as a negative value. Ø A + e- A- + energy F has the highest value of -339. 9. o Positive or less negative values indicate atom was “forced” to get electron, i. e. , energy was absorbed. o Most atoms so forced will spontaneously lose electron. Ø A + e- + energy A- Values are often listed as “(0)”.

Periodic Properties’ Trends • Electronegativity = ability to attract electron in a compound • F has highest value of 4. 0; others are relative. § Fluorine becomes most like a noble gas (filled valence shell = greater stability) when is F-. • Fr/Cs has lowest. § Alkali metals are energetically most stable when they lose their valence electron, not gain one.


Additions to Periodic Table • Noble gases – 1894: Ar; 1895: He; 1898: Kr & Xe; 1900 Rn • Lanthanides – Early 1900 s – Very similar in chemical & physical properties – Soft and shiny; reactivity like alkaline-earth metals • Actinides – All are radioactive; 1 st 4 are natural, rest synthetic – Those after uranium are “transuranium elements”.


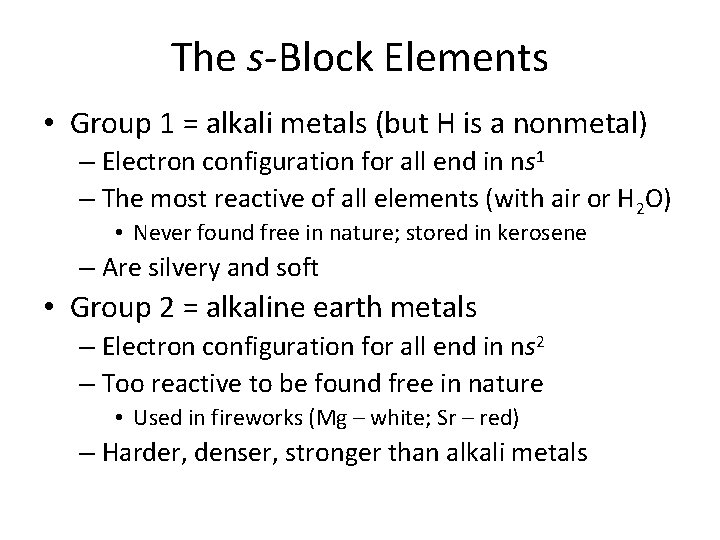
The s-Block Elements • Group 1 = alkali metals (but H is a nonmetal) – Electron configuration for all end in ns 1 – The most reactive of all elements (with air or H 2 O) • Never found free in nature; stored in kerosene – Are silvery and soft • Group 2 = alkaline earth metals – Electron configuration for all end in ns 2 – Too reactive to be found free in nature • Used in fireworks (Mg – white; Sr – red) – Harder, denser, stronger than alkali metals

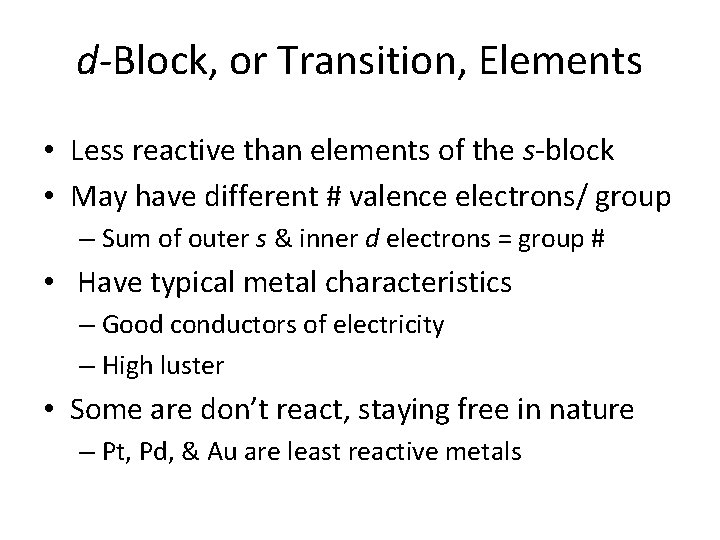
d-Block, or Transition, Elements • Less reactive than elements of the s-block • May have different # valence electrons/ group – Sum of outer s & inner d electrons = group # • Have typical metal characteristics – Good conductors of electricity – High luster • Some are don’t react, staying free in nature – Pt, Pd, & Au are least reactive metals

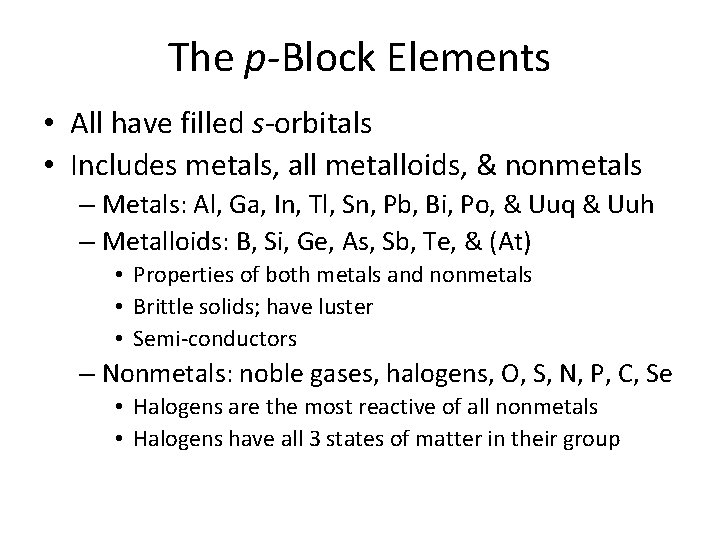
The p-Block Elements • All have filled s-orbitals • Includes metals, all metalloids, & nonmetals – Metals: Al, Ga, In, Tl, Sn, Pb, Bi, Po, & Uuq & Uuh – Metalloids: B, Si, Ge, As, Sb, Te, & (At) • Properties of both metals and nonmetals • Brittle solids; have luster • Semi-conductors – Nonmetals: noble gases, halogens, O, S, N, P, C, Se • Halogens are the most reactive of all nonmetals • Halogens have all 3 states of matter in their group


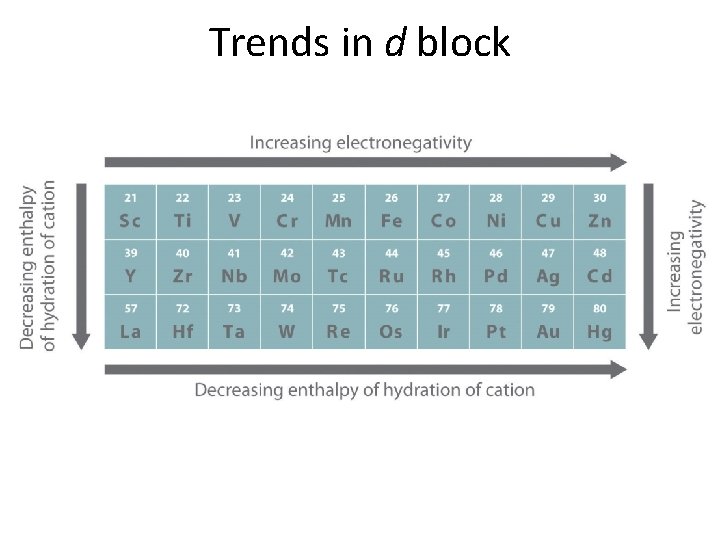
Trends for p- & d-blocks • Atomic radii: going right across period • Ionization energy (IE): going right across period & going down the group for the 1 st IE – Outer s electrons are less shielded by d electrons • Ion formation & radii: all lose ns 2 electrons 1 st – Ions of a 2+ charge in size across period • Electronegativity: {F = 4. 0; others are relative} – d block: all are 1. 1 – 2. 54 – f block: all are 1. 1 – 1. 5 – Inversely proportional to atomic radii for both.


Trends in d block
 Atomic radius and ionization energy
Atomic radius and ionization energy Zeus
Zeus Amanda rinehart
Amanda rinehart Chapter 6 the periodic table and periodic law
Chapter 6 the periodic table and periodic law Chapter 6 periodic table
Chapter 6 periodic table The periodic table and periodic law chapter 6
The periodic table and periodic law chapter 6 Chemistry january 2018 answers
Chemistry january 2018 answers Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law
Newton's first law Boyles law
Boyles law P=k/v
P=k/v How to find out group and period of an element
How to find out group and period of an element Grafo di holt riducibile
Grafo di holt riducibile Hobbs and holt 1976
Hobbs and holt 1976 Hobbs and holt token economy
Hobbs and holt token economy Grass grows hans haacke
Grass grows hans haacke Holt vidék elemzés
Holt vidék elemzés Holt world history the human journey
Holt world history the human journey Direction of dipole moment
Direction of dipole moment Gayne corporation's contribution margin ratio
Gayne corporation's contribution margin ratio Bari gongju
Bari gongju Amelia holt
Amelia holt Holt sociology the study of human relationships
Holt sociology the study of human relationships