The Pathoanatomy of Functional MR as It Relates


- Slides: 11

The Pathoanatomy of Functional MR as It Relates to Transcatheter Therapy Ted Feldman, M. D. , MSCAI FACC FESC Evanston Hospital CRT Cardiovascular Research Technologies Washington D. C. February 18 -21 st, 2017

Ted Feldman MD, MSCAI FACC FESC Disclosure Information The following relationships exist: Grant support: Abbott, BSC, Cardiokinetics, Corvia, Edwards, WL Gore Consultant: Abbott, BSC, Edwards, WL Gore Stock Options: Mitralign Off label use of products and investigational devices will be discussed in this presentation

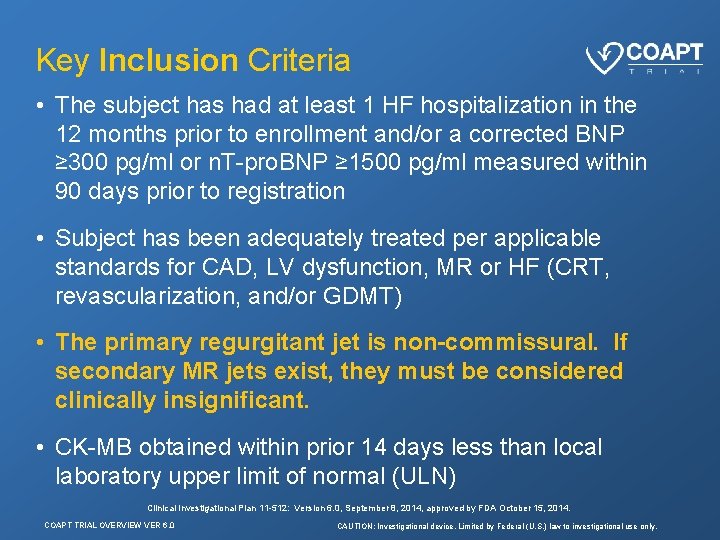
Key Inclusion Criteria • The subject has had at least 1 HF hospitalization in the 12 months prior to enrollment and/or a corrected BNP ≥ 300 pg/ml or n. T-pro. BNP ≥ 1500 pg/ml measured within 90 days prior to registration • Subject has been adequately treated per applicable standards for CAD, LV dysfunction, MR or HF (CRT, revascularization, and/or GDMT) • The primary regurgitant jet is non-commissural. If secondary MR jets exist, they must be considered clinically insignificant. • CK-MB obtained within prior 14 days less than local laboratory upper limit of normal (ULN) Clinical Investigational Plan 11 -512: Version 6. 0, September 8, 2014, approved by FDA October 15, 2014. COAPT TRIAL OVERVIEW VER 6. 0 CAUTION: Investigational device. Limited by Federal (U. S. ) law to investigational use only.

Key Exclusion Criteria • Leaflet anatomy which may preclude Mitra. Clip implantation, proper Mitra. Clip positioning on the leaflets, or sufficient MR reduction by the Mitra. Clip – Insufficient mobile leaflet available for grasping with the Mitra. Clip device – Evidence of calcification in the grasping area – Presence of a significant cleft in the grasping area – Lack of both primary and secondary chordal support in the grasping area – Leaflet mobility length < 1 cm • MV orifice area <4. 0 cm 2, confirmed by the Site 90 days prior to registration Clinical Investigational Plan 11 -512: Version 6. 0, September 8, 2014, approved by FDA October 15, 2014. COAPT TRIAL OVERVIEW VER 6. 0 CAUTION: Investigational device. Limited by Federal (U. S. ) law to investigational use only.

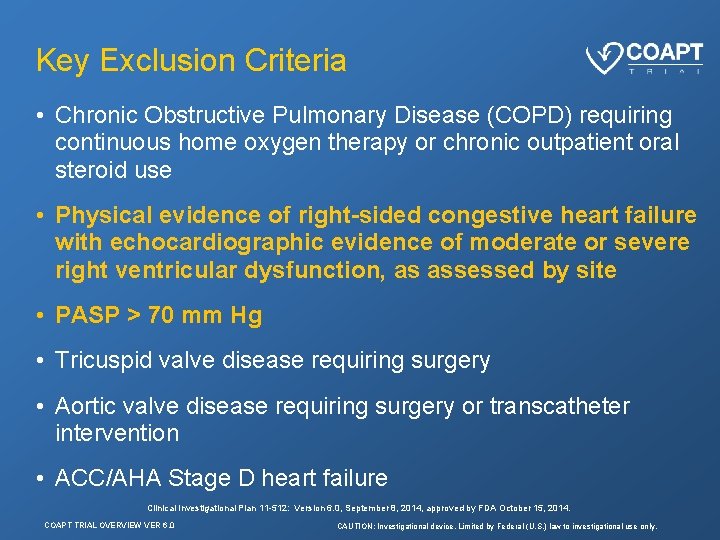
Key Exclusion Criteria • Chronic Obstructive Pulmonary Disease (COPD) requiring continuous home oxygen therapy or chronic outpatient oral steroid use • Physical evidence of right-sided congestive heart failure with echocardiographic evidence of moderate or severe right ventricular dysfunction, as assessed by site • PASP > 70 mm Hg • Tricuspid valve disease requiring surgery • Aortic valve disease requiring surgery or transcatheter intervention • ACC/AHA Stage D heart failure Clinical Investigational Plan 11 -512: Version 6. 0, September 8, 2014, approved by FDA October 15, 2014. COAPT TRIAL OVERVIEW VER 6. 0 CAUTION: Investigational device. Limited by Federal (U. S. ) law to investigational use only.

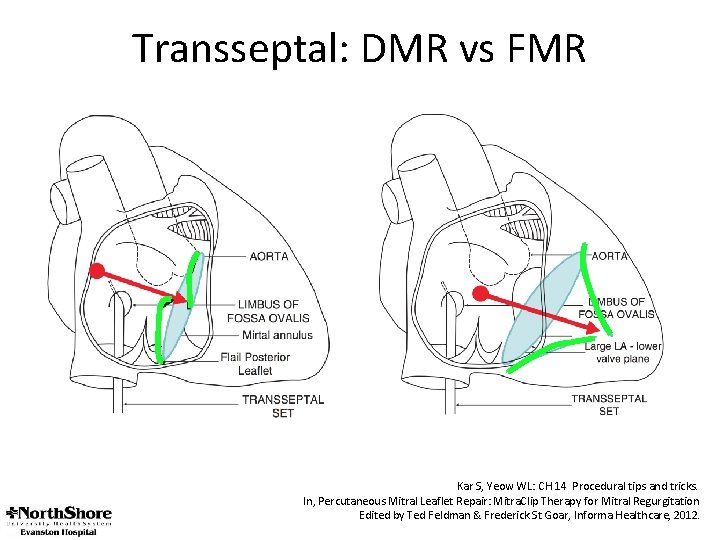
Transseptal: DMR vs FMR Kar S, Yeow WL: CH 14 Procedural tips and tricks. In, Percutaneous Mitral Leaflet Repair: Mitra. Clip Therapy for Mitral Regurgitation Edited by Ted Feldman & Frederick St Goar, Informa Healthcare, 2012.

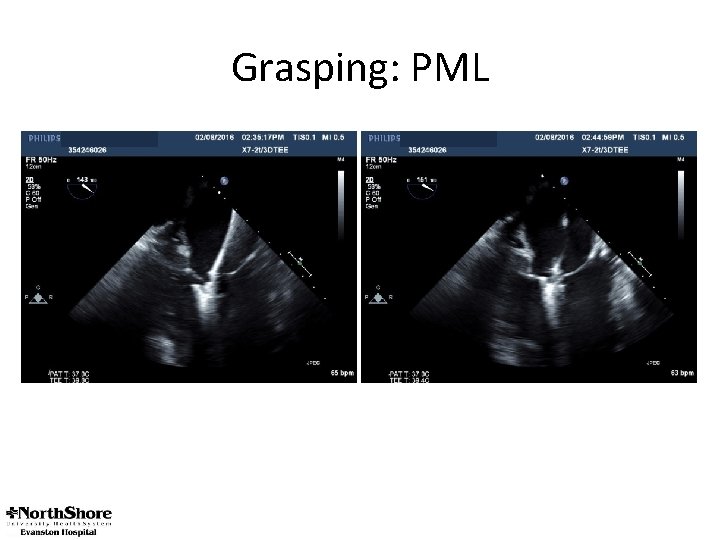
Grasping: PML

CARDIOBAND Edwards PASCAL Repair System

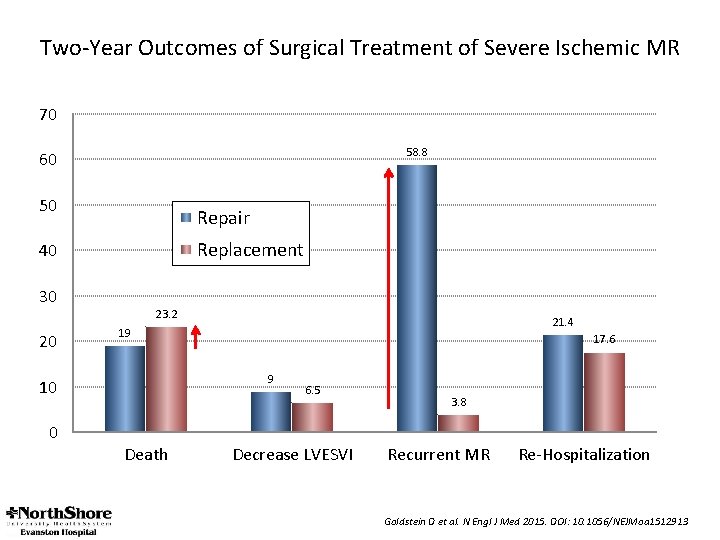
Two-Year Outcomes of Surgical Treatment of Severe Ischemic MR 70 58. 8 60 50 Repair Replacement 40 30 20 23. 2 17. 6 9 10 0 21. 4 19 Death 6. 5 Decrease LVESVI 3. 8 Recurrent MR Re-Hospitalization Goldstein D et al. N Engl J Med 2015. DOI: 10. 1056/NEJMoa 1512913

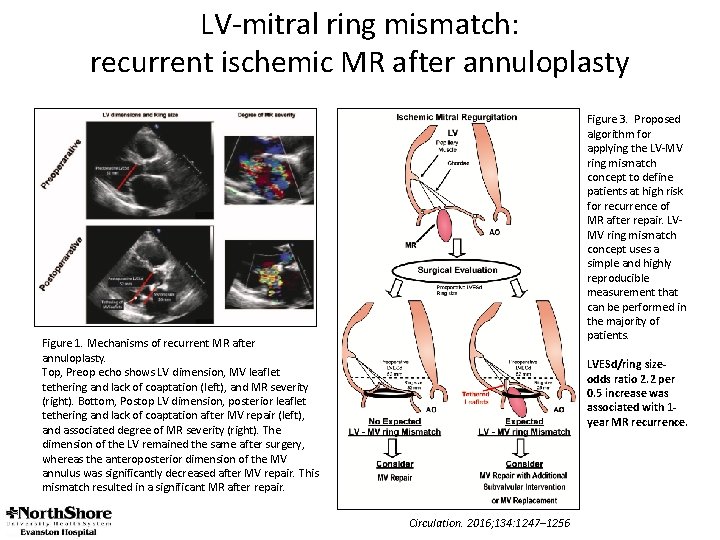
LV-mitral ring mismatch: recurrent ischemic MR after annuloplasty Figure 3. Proposed algorithm for applying the LV-MV ring mismatch concept to define patients at high risk for recurrence of MR after repair. LVMV ring mismatch concept uses a simple and highly reproducible measurement that can be performed in the majority of patients. Figure 1. Mechanisms of recurrent MR after annuloplasty. Top, Preop echo shows LV dimension, MV leaflet tethering and lack of coaptation (left), and MR severity (right). Bottom, Postop LV dimension, posterior leaflet tethering and lack of coaptation after MV repair (left), and associated degree of MR severity (right). The dimension of the LV remained the same after surgery, whereas the anteroposterior dimension of the MV annulus was significantly decreased after MV repair. This mismatch resulted in a significant MR after repair. LVESd/ring sizeodds ratio 2. 2 per 0. 5 increase was associated with 1 year MR recurrence. Circulation. 2016; 134: 1247– 1256

TMV Replacement • • Fixation/Anchoring/Migration PVL LVOT obstruction Hemolysis Calcium Apical access Transseptal access Philipp Blanke, MD