The Particle Model and Changes of State Unit





- Slides: 17

The Particle Model and Changes of State Unit 3 - Topic 5

Representing the 3 States of Matter 1. Divide the class into three (3) groups. 2. Group 1 – Arrange yourselves to demonstrate the arrangement and movement of particles in a solid. 3. Group 2 – Arrange yourselves to demonstrate the arrangement and movement of particles in a liquid. 4. Group 3 – Arrange yourselves to demonstrate the arrangement and movement of particles in a gas.

The 3 States of Water What are three states of water? How does water move from one state to another? What do you think happens to the particles as water moves from one state to another?

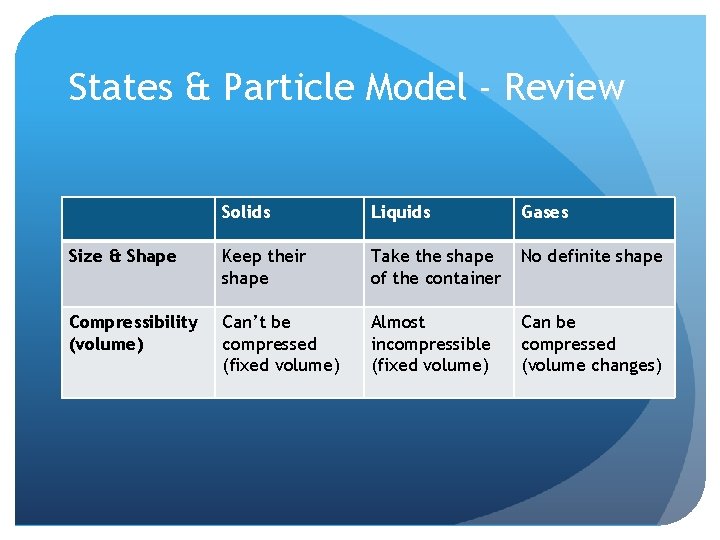
States & Particle Model - Review Solids Liquids Gases Size & Shape Keep their shape Take the shape of the container No definite shape Compressibility (volume) Can’t be compressed (fixed volume) Almost incompressible (fixed volume) Can be compressed (volume changes)

Add thermal energy… Substances expand by different amounts their temperatures rise differently change state

Add thermal energy… Substances expand by different amounts concrete vs. steel vs. glass (see page. 211)

Add thermal energy… Substances’ temperatures rise differently Given the same heat… Sand warms and cools quickly Water warms and cools slowly Due to (pg. 218): Heat capacity Specific heat capacity

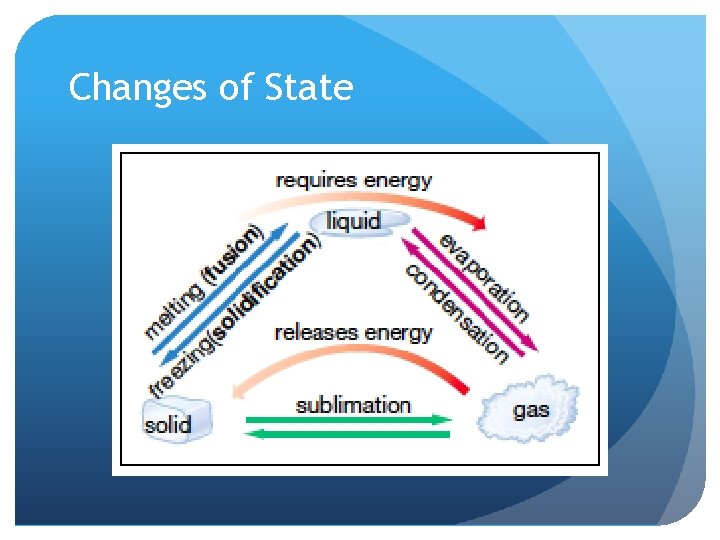
Add thermal energy… Substances change state: Melting (fusion) freezing (solidification) evaporation: condensation: sublimation: solid liquid solid liquid gas liquid gas solid gas

Changes of State

Temperature During Change of State Temperature constant = avg. speed of particles constant Speed of particles changes = temperature changes Arrangement of the particles changes Particles become less organized as their energy increases (just like students!) Particles become more organized as their energy drops

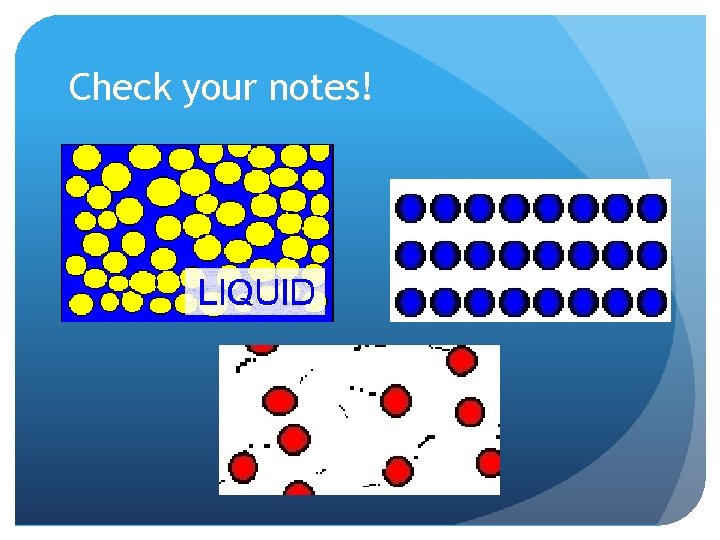
Check your notes! Solid Gas

Pure Substances Can exist in all 3 states of matter Examples water, hydrogen, gold Non – examples paints, plastics not able to melt and then return to a solid or gas form

Most substances not easy to study Hydrogen (H 2) a gas, even at coldest winter temperatures to make liquid H 2, cool H 2 gas to -235 o. C to make solid H 2, extreme cold temperatures and high pressures required

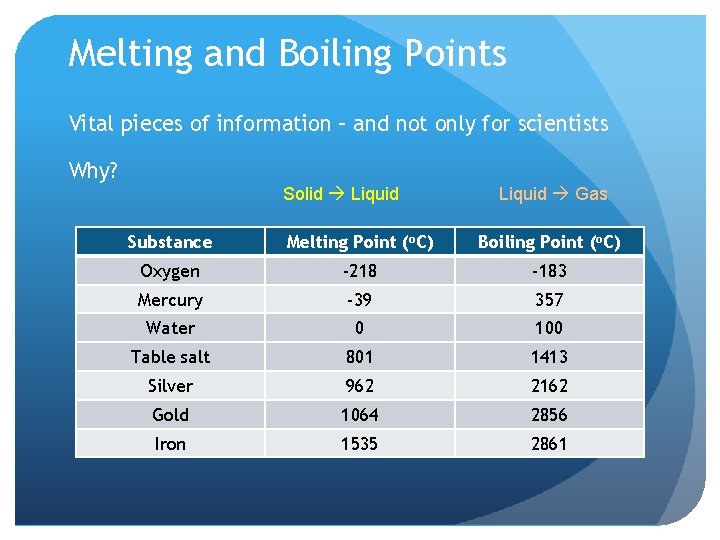
Melting and Boiling Points Vital pieces of information – and not only for scientists Why? Solid Liquid Gas Substance Melting Point (o. C) Boiling Point (o. C) Oxygen -218 -183 Mercury -39 357 Water 0 100 Table salt 801 1413 Silver 962 2162 Gold 1064 2856 Iron 1535 2861

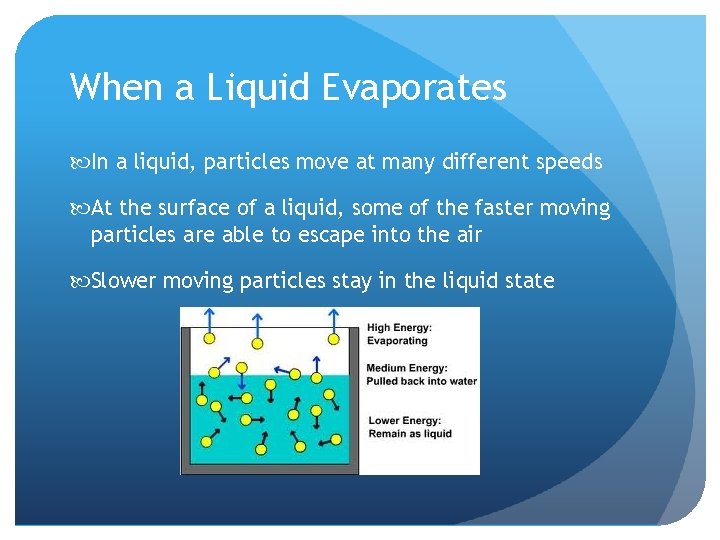
When a Liquid Evaporates In a liquid, particles move at many different speeds At the surface of a liquid, some of the faster moving particles are able to escape into the air Slower moving particles stay in the liquid state

When a Liquid Evaporates Slower motion = lower average energy = lower temperature As the higher energy particles leave the surface, the remaining liquid is cooler than the original liquid, which helps cool lower particles This phenomenon is called EVAPORATIVE COOLING

To summarize… Adding thermal energy to substances 6 changes of state Temperature constant during change of state Arrangement of particles changes during change of state Pure substances Evaporative cooling