The P Block Nitrogen and Group 5 P

The P Block Nitrogen and Group 5 P block group V 1

Group 5 • Is at the centre of the periodic table • Top of group, non-metals: – Nitrogen and Phosphorus • Bottom of group, metalloids (metallic elements with non-metal character): – Antimony and Bismuth P block group V 2
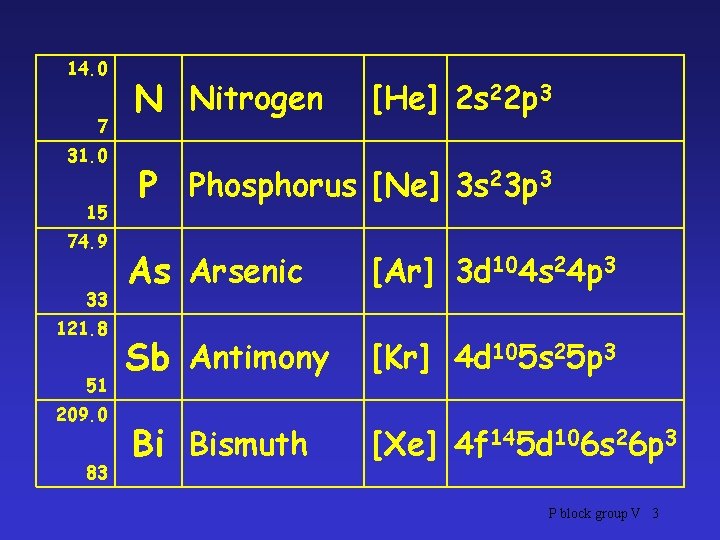
14. 0 7 31. 0 15 74. 9 33 121. 8 51 209. 0 83 N Nitrogen [He] 2 s 22 p 3 P Phosphorus [Ne] 3 s 23 p 3 As Arsenic [Ar] 3 d 104 s 24 p 3 Sb Antimony [Kr] 4 d 105 s 25 p 3 Bi Bismuth [Xe] 4 f 145 d 106 s 26 p 3 P block group V 3

Group 5 • Group 5 elements can form 3 covalent bonds – Sharing the 3 unpaired electrons – Gives compounds with oxidation state of +3 or – 3 for group 5 element • Each atom has a lone pair of electrons – Enables atoms to form dative covalent bonds – When they do this Group 5 elements can form some compounds with oxidation state of +5 for the Group 5 element P block group V 4

Nitrogen • Very abundant element • Very unreactive! • N 2 unreactive because of strong triple bond holding N’s together – Bond enthalpy of N N is +945 k. J mol-1 (Bond enthalpy of N-N is +158 k. J mol-1) – Hence most reaction of N 2 have high EA and require high T and catalysts to make them occur • Haber process- high T + Fe catalyst…. • Lightning or >2000ºC to make N 2 react with O 2 P block group V 5

Ammonia . . • Ammonia is nitrogen hydride, NH 3 • Lone pair not involved in bonding – Dative covalent bonds (dcb’s) possible P block group V 6
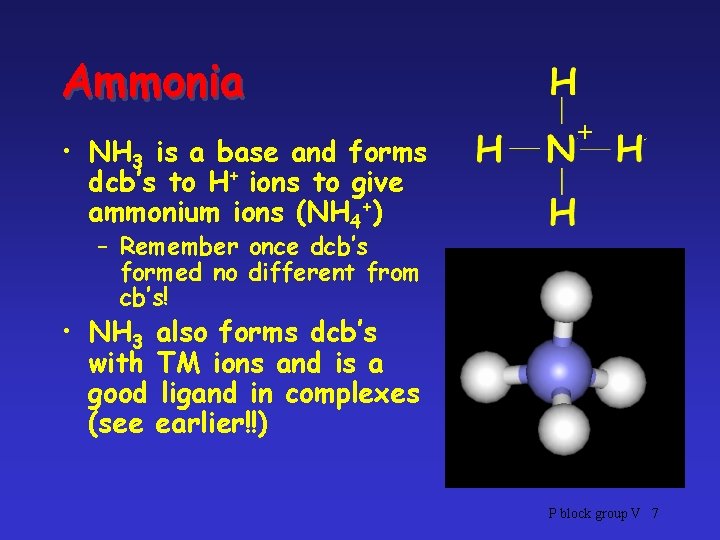
Ammonia • NH 3 is a base and forms dcb’s to H+ ions to give ammonium ions (NH 4+) + – Remember once dcb’s formed no different from cb’s! • NH 3 also forms dcb’s with TM ions and is a good ligand in complexes (see earlier!!) P block group V 7

Nitrogen Oxides Name / Formula • Nitrogen monoxide • NO Appearance Where it comes from • Colourless • Combustion gas, turns processes to brown • Thunderstorms NO 2 in air • Formed in the soil by denitrifying bacteria P block group V 8

Nitrogen Oxides Name / Formula • Nitrogen dioxide • NO 2 • Dinitrogen oxide • N 2 O Appearance Where it comes from • Brown gas • From oxidation of (toxic) NO in the atmosphere • Colourless gas • Formed in the soil by denitrifying bacteria P block group V 9

Nitrates • 2 Kinds of nitrate ions involved in the nitrogen cycle: – Nitrate(III), NO 2– Nitrate(V), NO 3 - • Both are called nitrates, but distinguished by showing OS of N P block group V 10
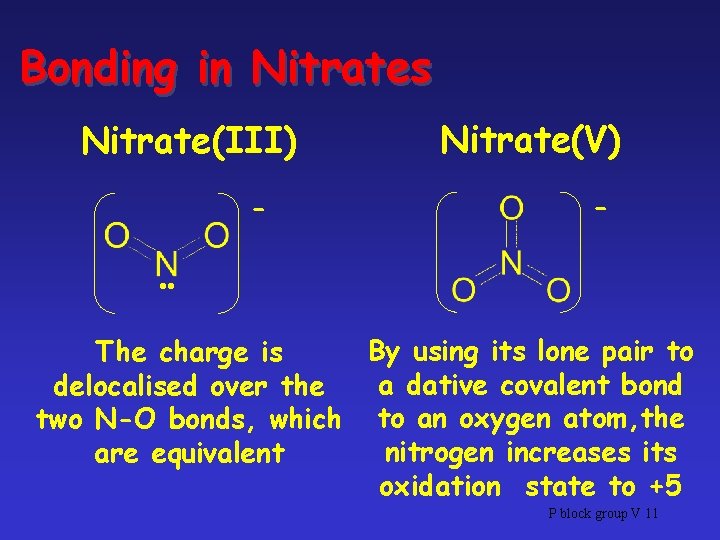
Bonding in Nitrates Nitrate(III) - Nitrate(V) - • • By using its lone pair to The charge is a dative covalent bond delocalised over the two N-O bonds, which to an oxygen atom, the nitrogen increases its are equivalent oxidation state to +5 P block group V 11
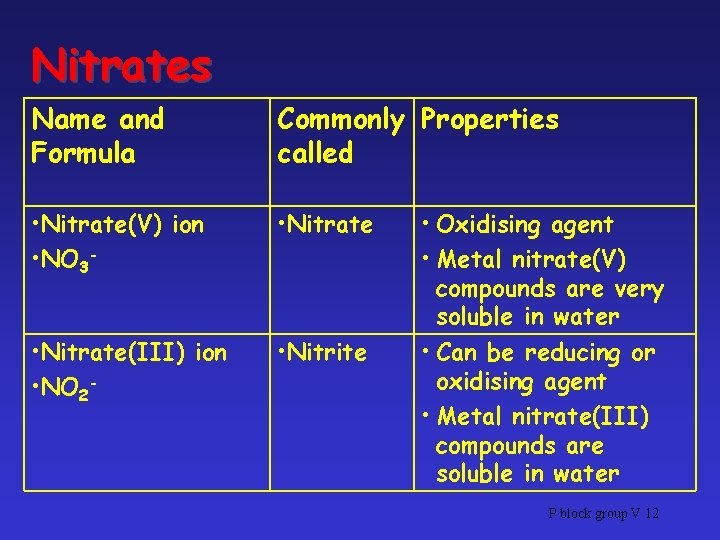
Nitrates Name and Formula Commonly Properties called • Nitrate(V) ion • NO 3 - • Nitrate(III) ion • NO 2 - • Nitrite • Oxidising agent • Metal nitrate(V) compounds are very soluble in water • Can be reducing or oxidising agent • Metal nitrate(III) compounds are soluble in water P block group V 12
- Slides: 12