The overall goal Determine the concentration of salt


- Slides: 17

The overall goal…. . Determine the concentration of salt in the Atlantic Ocean Is your determination correct (accurate)? How sure are you (precision)? Is the concentration sufficient for the lobsters to survive? Google Earth, Hancock, Maine (a picture of Mom and Pop)

Measurement: An Investigation of Mass, Volume, Density, Accuracy, and Precision goals: 1. to determine the density of salt water samples of known salt concentration 2. to determine the relationship between salt concentration and density (graph) 3. to use the relationship b/w salt concentration and density to determine the salt concentration in a sample of the Atlantic Ocean 4. to address accuracy and precision for all measurements and calculations ____________________________________ Density # of pieces in a given space ______________________ dense not so dense

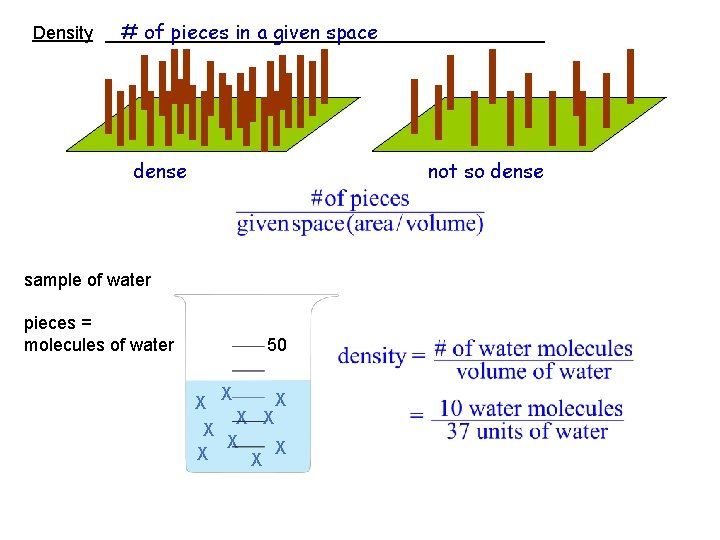
# of pieces in a given space Density ______________________ dense not so dense sample of water pieces = molecules of water 50 X X X X X

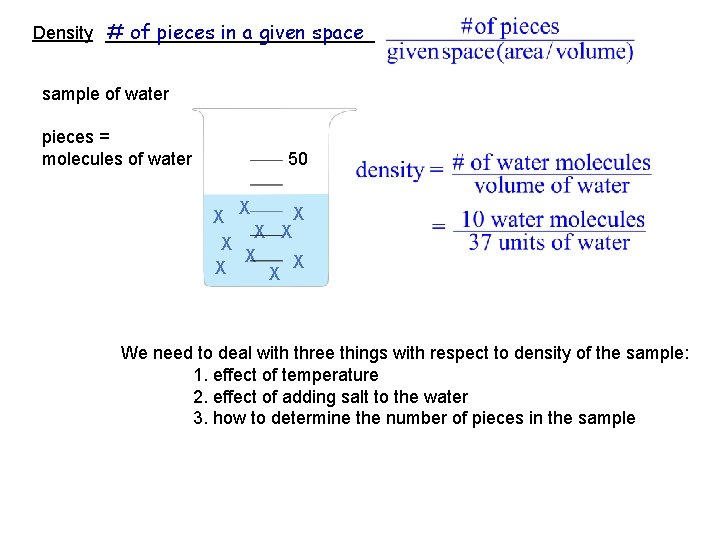
# of pieces in a given space Density ______________ sample of water pieces = molecules of water 50 X X X X X We need to deal with three things with respect to density of the sample: 1. effect of temperature 2. effect of adding salt to the water 3. how to determine the number of pieces in the sample

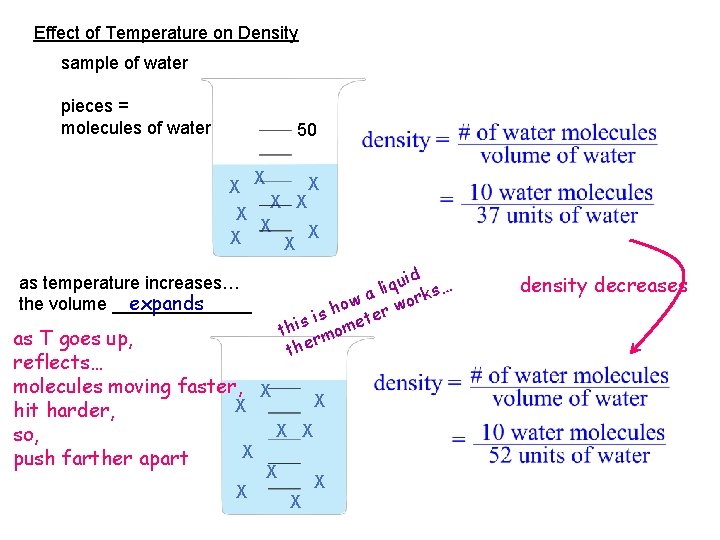
Effect of Temperature on Density sample of water pieces = molecules of water 50 X X X X X d qui s… i l k a ow r wor h s is mete i h t rmo the as temperature increases… expands the volume _______ as T goes up, reflects… molecules moving faster, X X X hit harder, X X so, X push farther apart X X density decreases

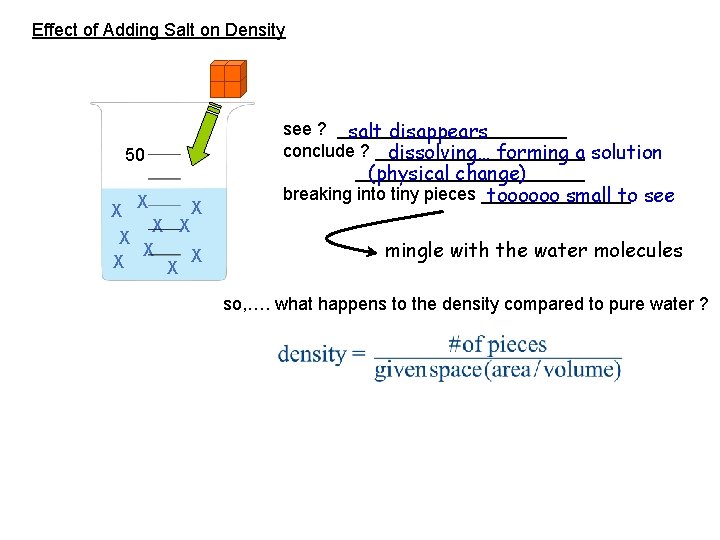
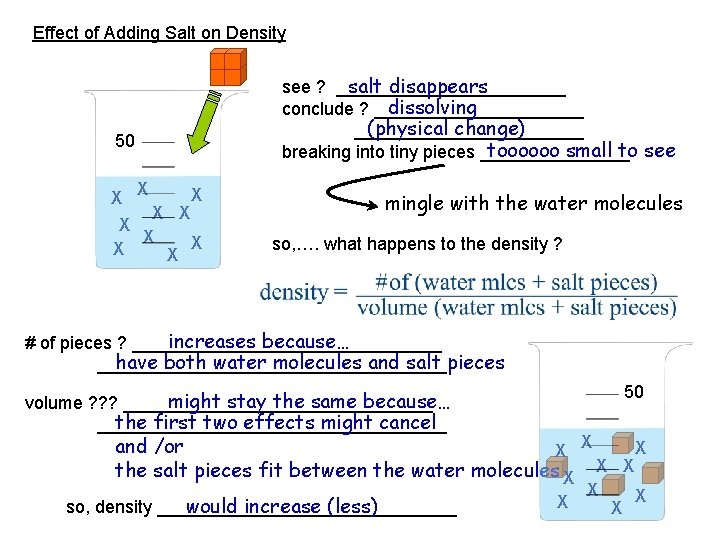
Effect of Adding Salt on Density 50 X X X X X see ? ____________ salt disappears conclude ? ___________ dissolving… forming a solution ____________ (physical change) breaking into tiny pieces ________ toooooo small to see mingle with the water molecules so, …. what happens to the density compared to pure water ?

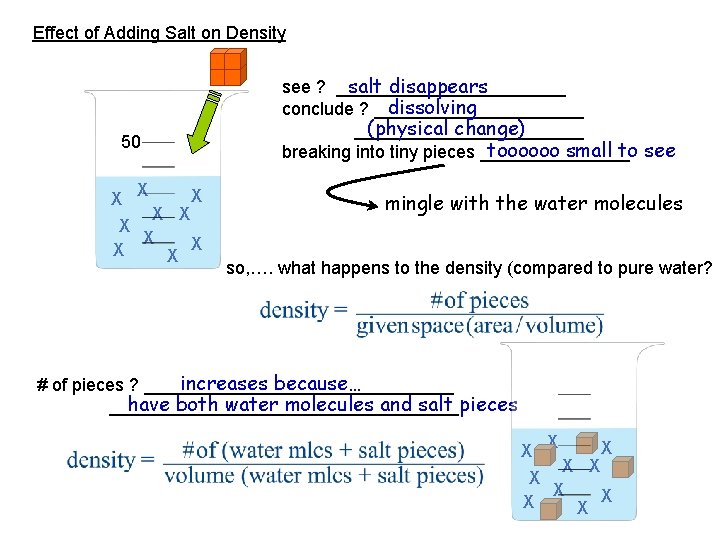
Effect of Adding Salt on Density salt disappears see ? ____________ dissolving conclude ? ___________ (physical change) ____________ toooooo small to see breaking into tiny pieces ________ 50 X X X X X mingle with the water molecules so, …. what happens to the density (compared to pure water? increases because… # of pieces ? ________________ have both water molecules and salt pieces __________________ X X X X X

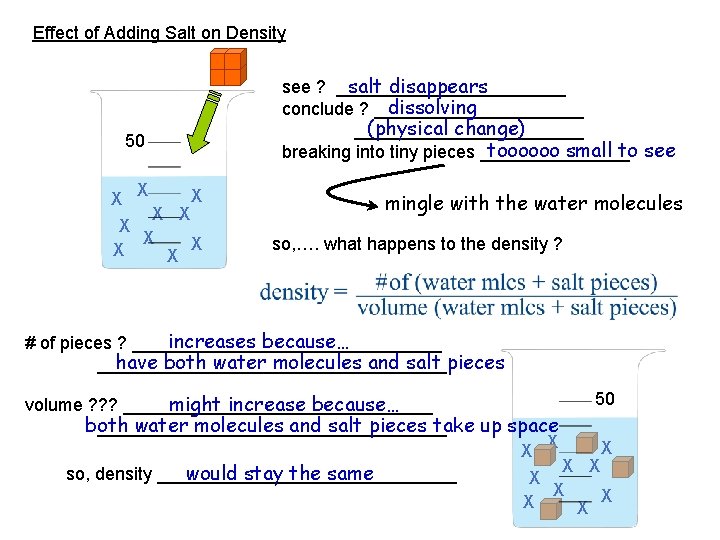
Effect of Adding Salt on Density salt disappears see ? ____________ dissolving conclude ? ___________ (physical change) ____________ toooooo small to see breaking into tiny pieces ________ 50 X X X X X mingle with the water molecules so, …. what happens to the density ? increases because… # of pieces ? ________________ have both water molecules and salt pieces __________________ 50 might increase because… volume ? ? ? ________________ both water molecules and salt pieces take up space __________________ X X X would stay the same so, density _______________ X X X

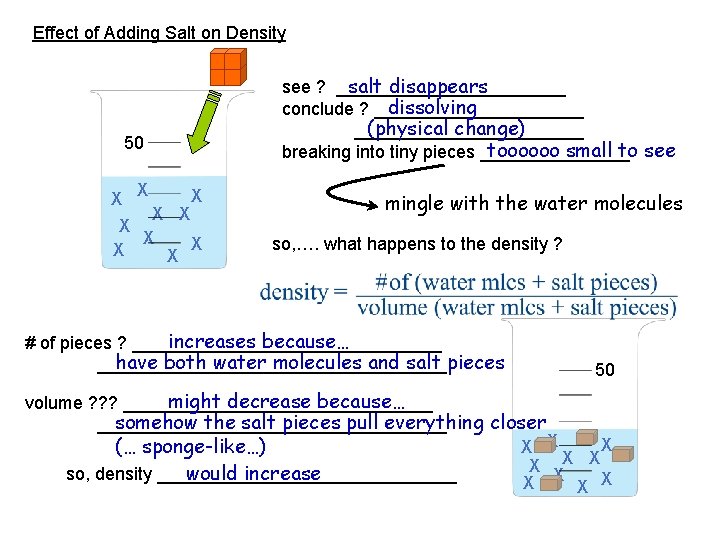
Effect of Adding Salt on Density salt disappears see ? ____________ dissolving conclude ? ___________ (physical change) ____________ toooooo small to see breaking into tiny pieces ________ 50 X X X X X mingle with the water molecules so, …. what happens to the density ? increases because… # of pieces ? ________________ have both water molecules and salt pieces __________________ 50 might decrease because… volume ? ? ? ________________ somehow the salt pieces pull everything closer __________________ X (… sponge-like…) X X X would increase so, density _______________ X X X

Effect of Adding Salt on Density salt disappears see ? ____________ dissolving conclude ? ___________ (physical change) ____________ toooooo small to see breaking into tiny pieces ________ 50 X X X X X mingle with the water molecules so, …. what happens to the density ? increases because… # of pieces ? ________________ have both water molecules and salt pieces __________________ 50 might stay the same because… volume ? ? ? ________________ the first two effects might cancel __________________ and /or X X X the salt pieces fit between the water molecules X X X would increase (less) so, density _______________ X X

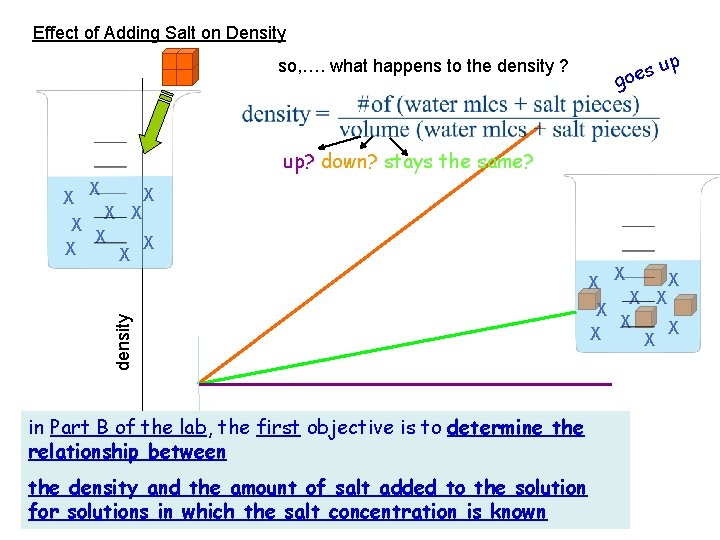
Effect of Adding Salt on Density so, …. what happens to the density ? p su goe up? down? stays the same? X X X density X X X X in Part B of the lab, the first objective is to determine the relationship between the density and the amount of salt added to the solution amount of salt added 0 for solutions in which the salt concentration is known X X X X X

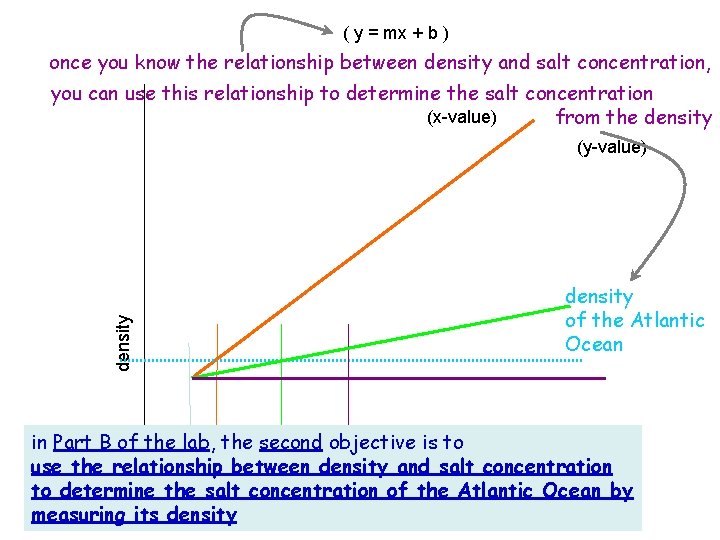
( y = mx + b ) once you know the relationship between density and salt concentration, you can use this relationship to determine the salt concentration (x-value) from the density (y-value) density of the Atlantic Ocean in Part B of the lab, the second objective is to use the relationship between density and salt concentration to determine the salt concentration of the Atlantic Ocean by amount of salt added 0 measuring its density

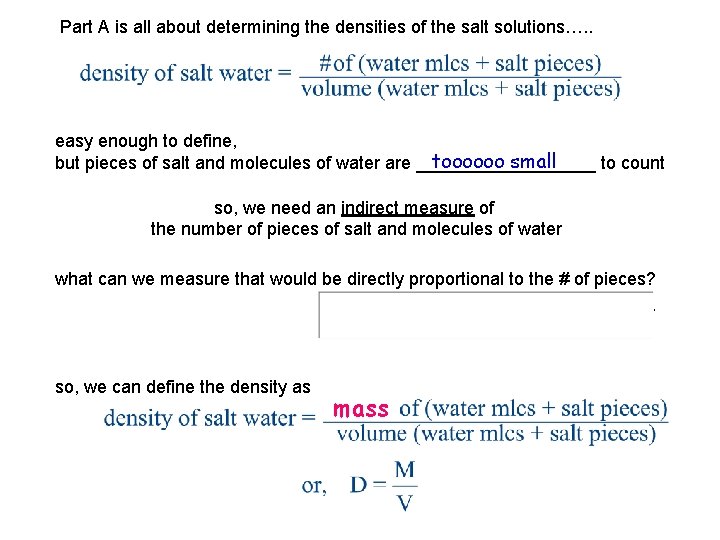
Part A is all about determining the densities of the salt solutions…. . easy enough to define, toooooo small but pieces of salt and molecules of water are _________ to count so, we need an indirect measure of the number of pieces of salt and molecules of water what can we measure that would be directly proportional to the # of pieces? ____________ so, we can define the density as mass

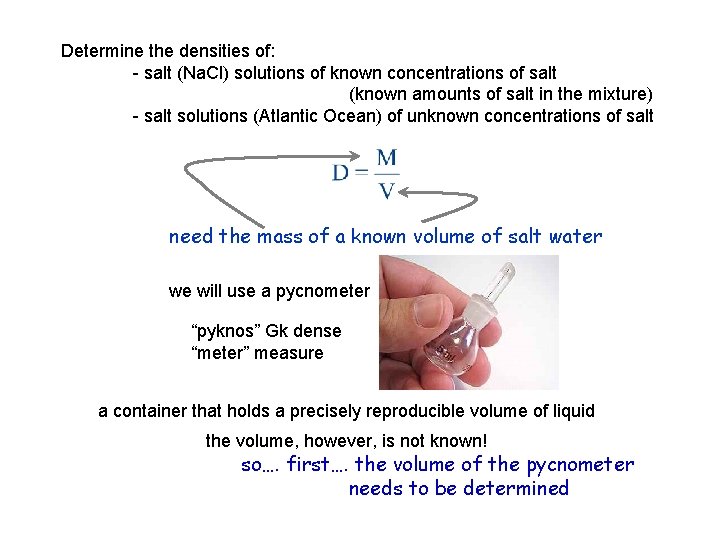
Determine the densities of: - salt (Na. Cl) solutions of known concentrations of salt (known amounts of salt in the mixture) - salt solutions (Atlantic Ocean) of unknown concentrations of salt need the mass of a known volume of salt water we will use a pycnometer “pyknos” Gk dense “meter” measure a container that holds a precisely reproducible volume of liquid the volume, however, is not known! so…. first…. the volume of the pycnometer needs to be determined

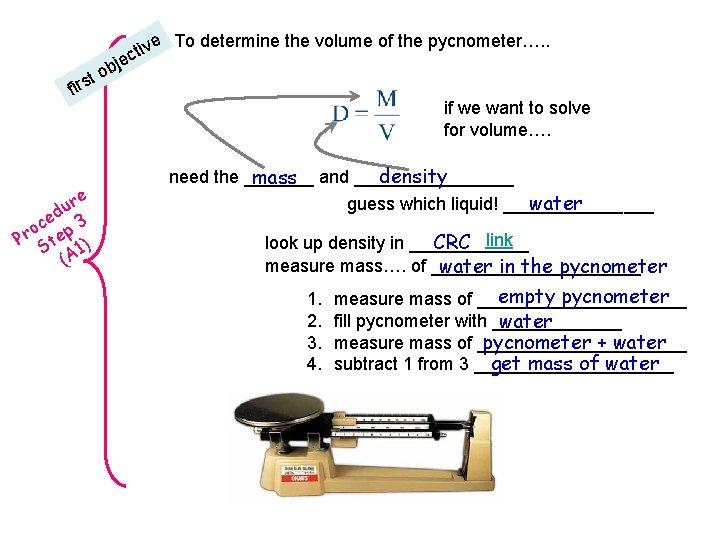
e To determine the volume of the pycnometer…. . b o rst fi re u ed 3 c o Pr tep ) S A 1 ( tiv c e j if we want to solve for volume…. density need the _______ mass and ________ water guess which liquid! ________ CRC link look up density in ______ measure mass…. of ___________ water in the pycnometer 1. 2. 3. 4. empty pycnometer measure mass of ___________ fill pycnometer with _______ water measure mass of ___________ pycnometer + water get mass of water subtract 1 from 3 __________

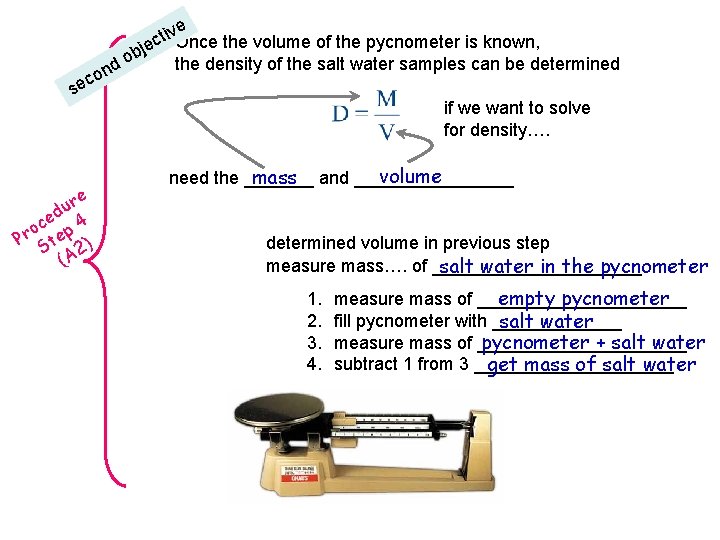
co se re u ed 4 c o Pr tep ) S A 2 ( e tiv. Once the volume of the pycnometer is known, c bje o the density of the salt water samples can be determined nd if we want to solve for density…. volume mass and ________ need the _______ determined volume in previous step measure mass…. of ___________ salt water in the pycnometer 1. 2. 3. 4. empty pycnometer measure mass of ___________ fill pycnometer with _______ salt water measure mass of ___________ pycnometer + salt water subtract 1 from 3 __________ get mass of salt water

Determine the density of salt water samples: One of four standards (known concentration of salt) 1. 30. 0 ppt Na. Cl by mass 2. 60. 0 ppt Na. Cl by mass 3. 90. 0 ppt Na. Cl by mass 4. 120. 0 ppt Na. Cl by mass and a sample of the Atlantic Ocean (Mom and Pop’s front yard, Hancock, Maine) parts per thousand [parts] ppt stands for __________________ (per cent is pph ________________) parts per hundred [parts] grams “parts” (by mass) ___________ so, an aqueous solution of 30. 0 ppt Na. Cl means