The Organic Chemistry of EnzymeCatalyzed Reactions Chapter 3
















![Reaction of Yeast Alcohol Dehydrogenase (YADH) with (A) [1, 1 -2 H 2]ethanol and Reaction of Yeast Alcohol Dehydrogenase (YADH) with (A) [1, 1 -2 H 2]ethanol and](https://slidetodoc.com/presentation_image_h2/956856d1171ecfa471fe4a5cc24c88be/image-34.jpg)
![Reaction of YADH with (A) [4 -2 H]NAD 2 H Prepared in Scheme 3. Reaction of YADH with (A) [4 -2 H]NAD 2 H Prepared in Scheme 3.](https://slidetodoc.com/presentation_image_h2/956856d1171ecfa471fe4a5cc24c88be/image-35.jpg)


![Urocanase Reaction Run with a [13 C] Pseudo-substrate “substrate” exchangeable proton apo-urocanase reconstituted with Urocanase Reaction Run with a [13 C] Pseudo-substrate “substrate” exchangeable proton apo-urocanase reconstituted with](https://slidetodoc.com/presentation_image_h2/956856d1171ecfa471fe4a5cc24c88be/image-44.jpg)





















![Iron-sulfur Clusters [2 Fe-2 S] [3 Fe-4 S] [4 Fe-4 S] 1 electron and Iron-sulfur Clusters [2 Fe-2 S] [3 Fe-4 S] [4 Fe-4 S] 1 electron and](https://slidetodoc.com/presentation_image_h2/956856d1171ecfa471fe4a5cc24c88be/image-119.jpg)

- Slides: 125

The Organic Chemistry of Enzyme-Catalyzed Reactions Chapter 3 Reduction and Oxidation

Redox Without a Coenzyme Internal redox reaction

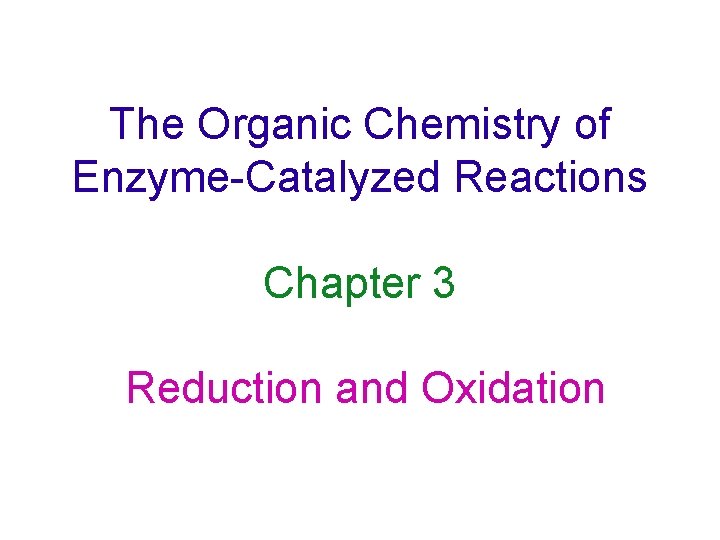
Reaction Catalyzed by Glyoxalase Scheme 3. 1 methylglyoxal lactic acid Looks like a Cannizzaro reaction

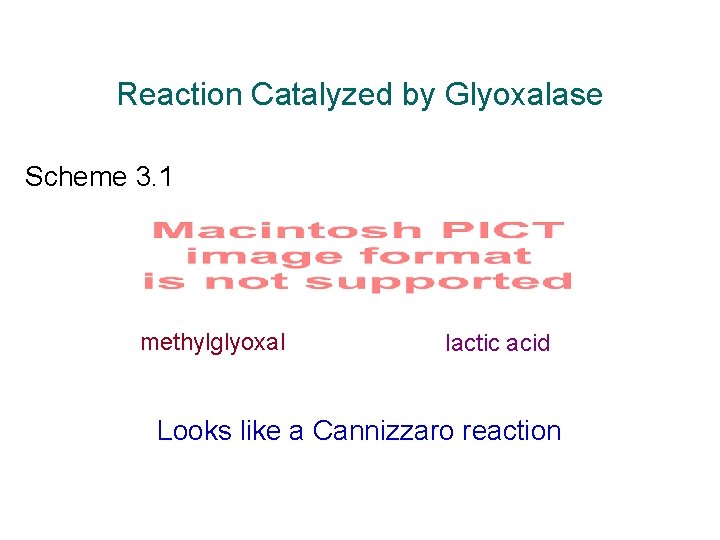
Cannizzaro Reaction Mechanism Scheme 3. 2

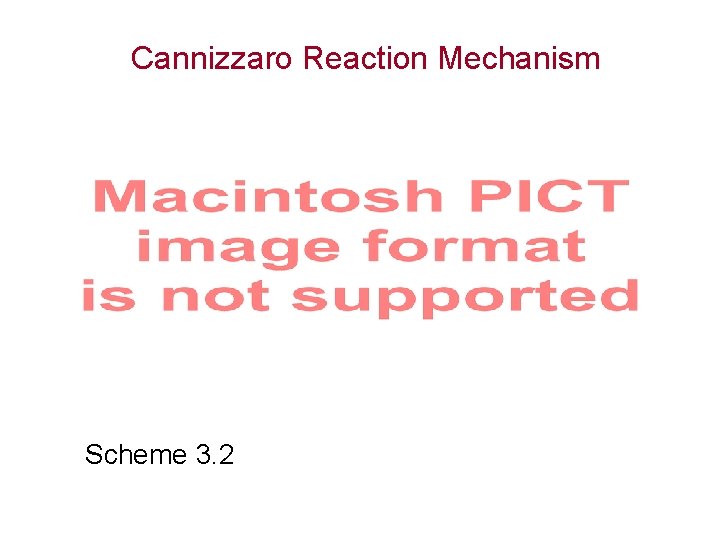
Reactions Catalyzed by Glyoxalase I and Glyoxalase II reduced glutathione Scheme 3. 3 oxidized

Glutathione (GSH)

Hydride Mechanism for Glyoxalase reduced Scheme 3. 4 Intramolecular Cannizzaro reaction oxidized

• Evidence for a hydride mechanism - when run in 3 H 2 O, lactate contains less than 4% tritium • NMR experiment provided evidence for a proton transfer mechanism: Enzyme reaction followed by NMR – At 25 °C in 2 H 2 O, 15% deuterium was incorporated – At 35 °C, 22% deuterium was incorporated

Enediol Mechanism for Glyoxalase cis-enediol Scheme 3. 5

Reaction of Glyoxalase with Fluoromethylglyoxal Another test for the mechanism Scheme 3. 6 same oxidation state

Hydride Mechanism for the Reaction of Glyoxalase with Fluoromethylglyoxal Scheme 3. 7

Enediol Mechanism for the Reaction of Glyoxalase with Fluoromethylglyoxal Scheme 3. 8

Hydride Mechanism for the Reaction of Glyoxalase with Deuterated Fluoromethylglyoxal Scheme 3. 9 deuterium isotope effect F- loss decreased

Enediol Mechanism for the Reaction of Glyoxalase with Deuterated Fluoromethylglyoxal Scheme 3. 10 F- loss increased deuterium isotope effect

increased F- loss supports enediol mechanism

Redox Reactions that Require Coenzymes Nicotinamide Coenzymes (Pyridine Nucleotides) • Pyridine nucleotide coenzymes include nicotinamide adenine dinucleotide (NAD+, 3. 10 a), nicotinamide adenine dinucleotide phosphate (NADP+, 3. 10 b), and reduced nicotinamide adenine dinucleotide phosphate (NADPH, 3. 11 b)

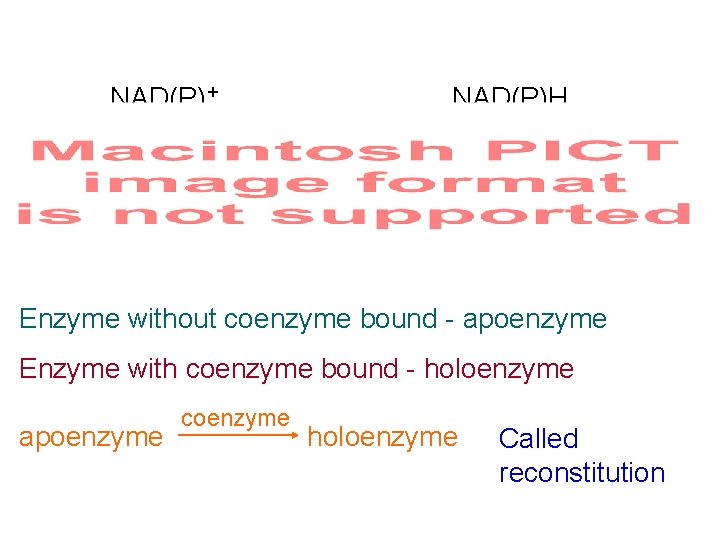
NAD(P)+ NAD(P)H Enzyme without coenzyme bound - apoenzyme Enzyme with coenzyme bound - holoenzyme apoenzyme coenzyme holoenzyme Called reconstitution

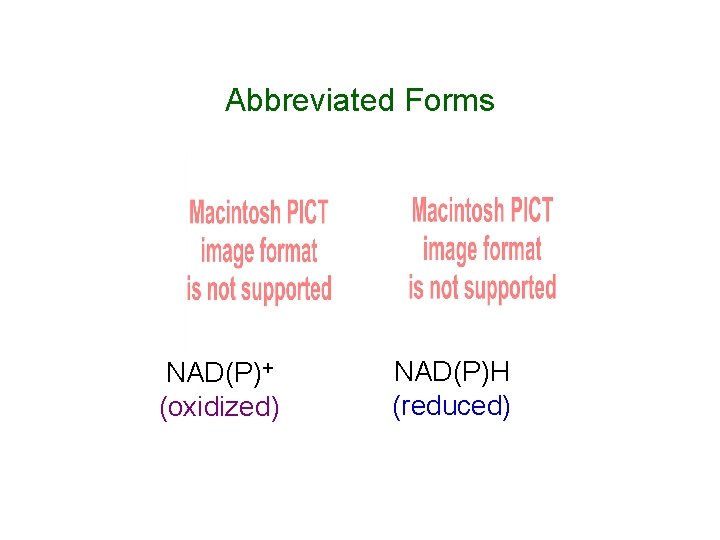
Abbreviated Forms NAD(P)+ (oxidized) NAD(P)H (reduced)

• Coenzymes typically derived from vitamins (compounds essential to our health, but not biosynthesized) • Pyridine nucleotide coenzymes derived from nicotinic acid (vitamin B 3, also known as niacin)

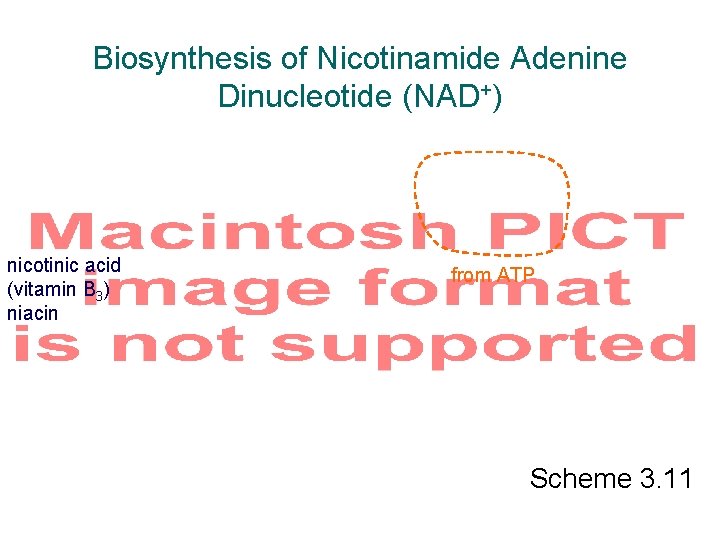
Biosynthesis of Nicotinamide Adenine Dinucleotide (NAD+) nicotinic acid (vitamin B 3) niacin from ATP Scheme 3. 11

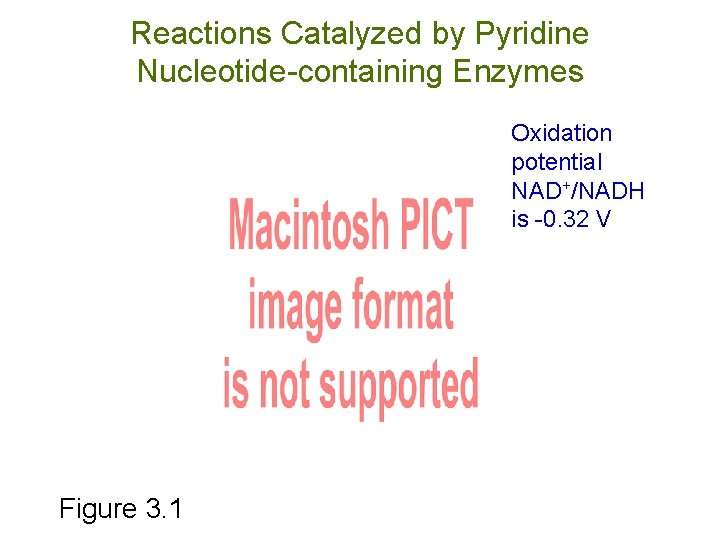
Reactions Catalyzed by Pyridine Nucleotide-containing Enzymes Oxidation potential NAD+/NADH is -0. 32 V Figure 3. 1

Reactions Catalyzed by Alcohol Dehydrogenases Mechanism Scheme 3. 12 In 3 H 2 O, no 3 H in NAD(P)H Hydride mechanism

Reaction Catalyzed by Alcohol Dehydrogenases Using Labeled Alcohol Scheme 3. 13 No *H found in H 2 O Supports hydride mechanism

Test for a radical intermediate Cyclopropylcarbinyl Radical Rearrangement Scheme 3. 14

Test for the Formation of a Radical Intermediate with Lactate Dehydrogenase Scheme 3. 15 No ring cleavage - evidence against radical mechanism

Chemical Model for the Potential Formation of a Cyclopropylcarbinyl Radical during the Lactate Dehydrogenase-catalyzed Reaction Scheme 3. 16 Should have seen ring opening in the enzyme reaction if a cyclopropylcarbinyl radical formed

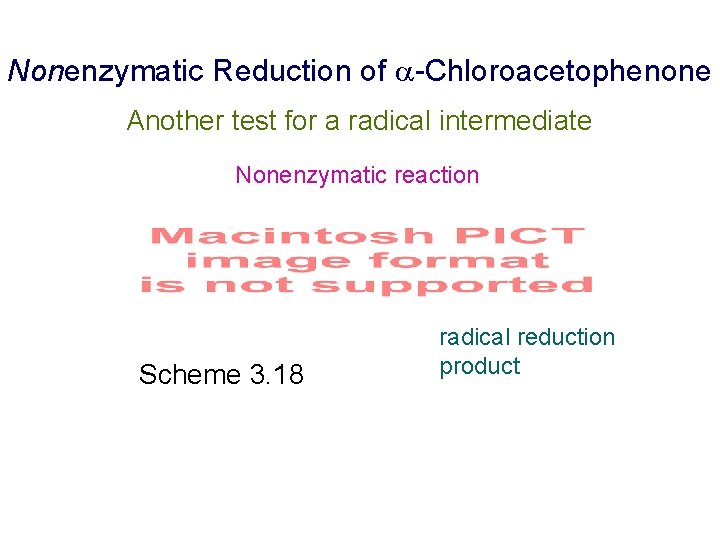
Nonenzymatic Reduction of -Chloroacetophenone Another test for a radical intermediate Nonenzymatic reaction Scheme 3. 18 radical reduction product

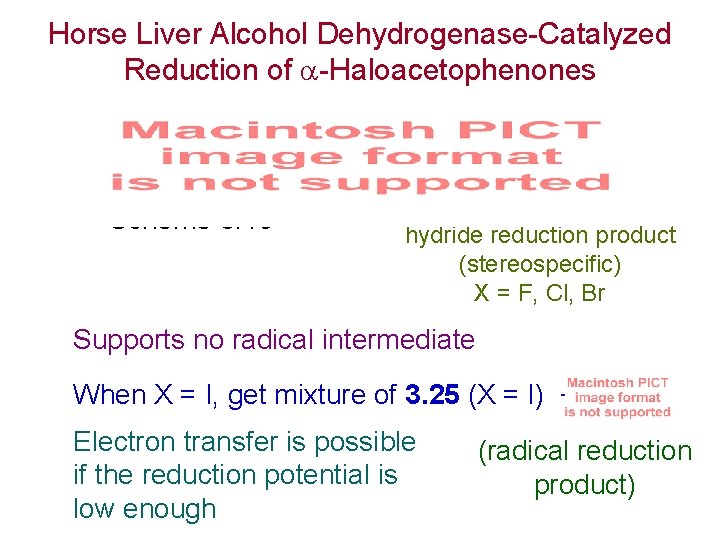
Horse Liver Alcohol Dehydrogenase-Catalyzed Reduction of -Haloacetophenones Scheme 3. 19 hydride reduction product (stereospecific) X = F, Cl, Br Supports no radical intermediate When X = I, get mixture of 3. 25 (X = I) + Electron transfer is possible if the reduction potential is low enough (radical reduction product)

Stereochemistry An atom is prochiral if by changing one of its substituents, it changes from achiral to chiral

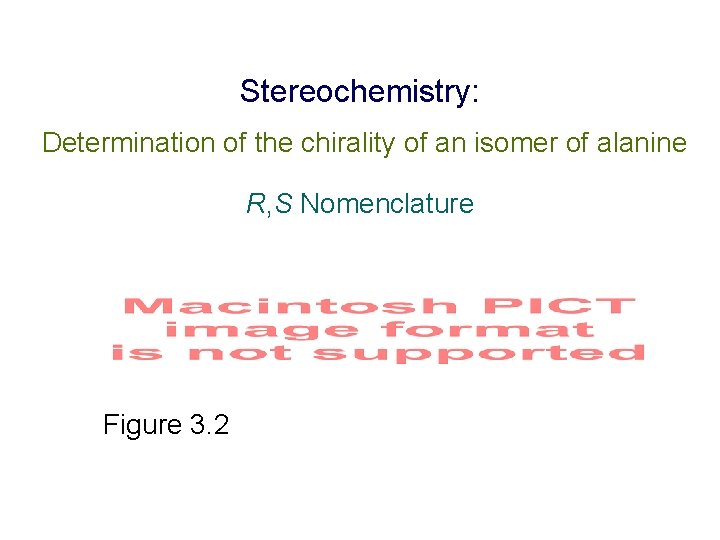
Stereochemistry: Determination of the chirality of an isomer of alanine R, S Nomenclature Figure 3. 2

Determination of Prochirality Figure 3. 3

Determination of sp 2 Carbon Chirality • Determine the priorities of the three substituents attached to the sp 2 carbon according to the R, S rules • If the priority sequence is clockwise looking down from top, then the top is the re face; if it is counterclockwise, then it is the si face

Determination of Carbonyl and Alkene (sp 2) Chirality Figure 3. 4
![Reaction of Yeast Alcohol Dehydrogenase YADH with A 1 1 2 H 2ethanol and Reaction of Yeast Alcohol Dehydrogenase (YADH) with (A) [1, 1 -2 H 2]ethanol and](https://slidetodoc.com/presentation_image_h2/956856d1171ecfa471fe4a5cc24c88be/image-34.jpg)
Reaction of Yeast Alcohol Dehydrogenase (YADH) with (A) [1, 1 -2 H 2]ethanol and NAD+ and (B) Ethanol and [4 -2 H]NAD+ Scheme 3. 20
![Reaction of YADH with A 4 2 HNAD 2 H Prepared in Scheme 3 Reaction of YADH with (A) [4 -2 H]NAD 2 H Prepared in Scheme 3.](https://slidetodoc.com/presentation_image_h2/956856d1171ecfa471fe4a5cc24c88be/image-35.jpg)
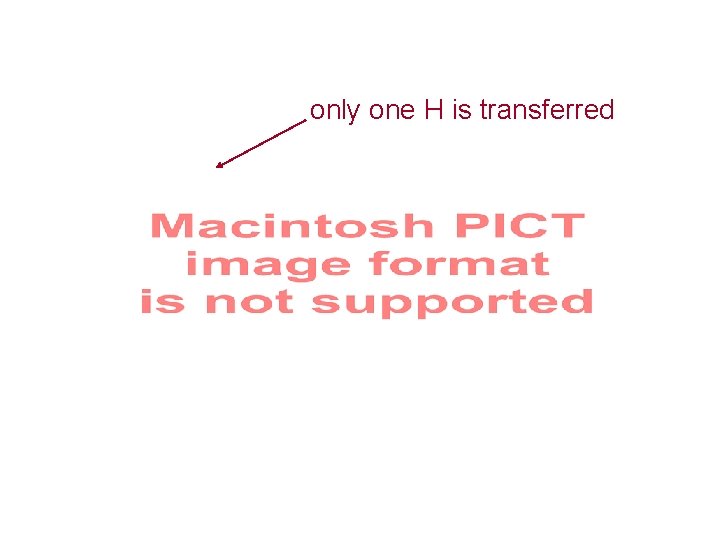
Reaction of YADH with (A) [4 -2 H]NAD 2 H Prepared in Scheme 3. 20 A; (B) Reaction of YADH with [4 -2 H]NAD 2 H Prepared in Scheme 3. 20 B; (C) Reaction of YADH with 3. 28 and NAD+ No 2 H No H Scheme 3. 21 stereospecific

only one H is transferred re-face

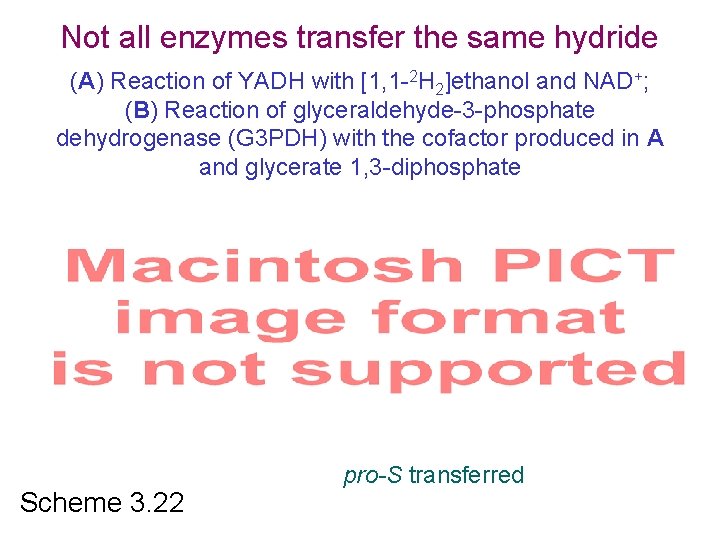
Not all enzymes transfer the same hydride (A) Reaction of YADH with [1, 1 -2 H 2]ethanol and NAD+; (B) Reaction of glyceraldehyde-3 -phosphate dehydrogenase (G 3 PDH) with the cofactor produced in A and glycerate 1, 3 -diphosphate pro-R Scheme 3. 22 pro-S transferred

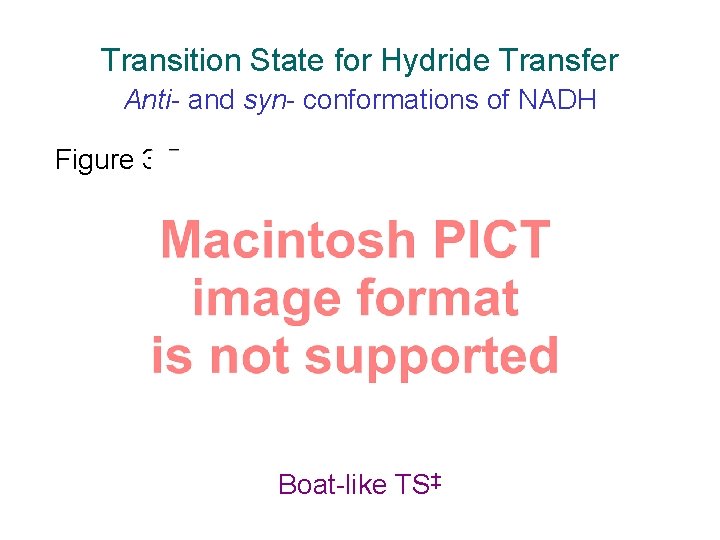
Transition State for Hydride Transfer Anti- and syn- conformations of NADH Figure 3. 5 syn-axial electrons assist Boat-like TS‡

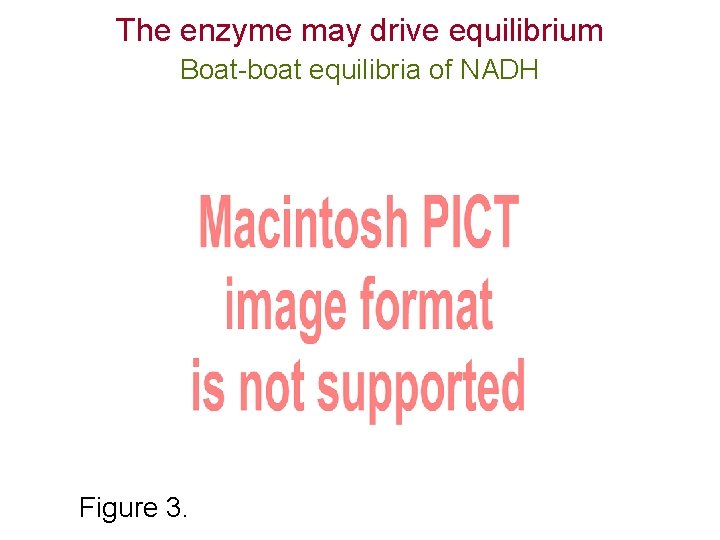
The enzyme may drive equilibrium Boat-boat equilibria of NADH Figure 3. 6

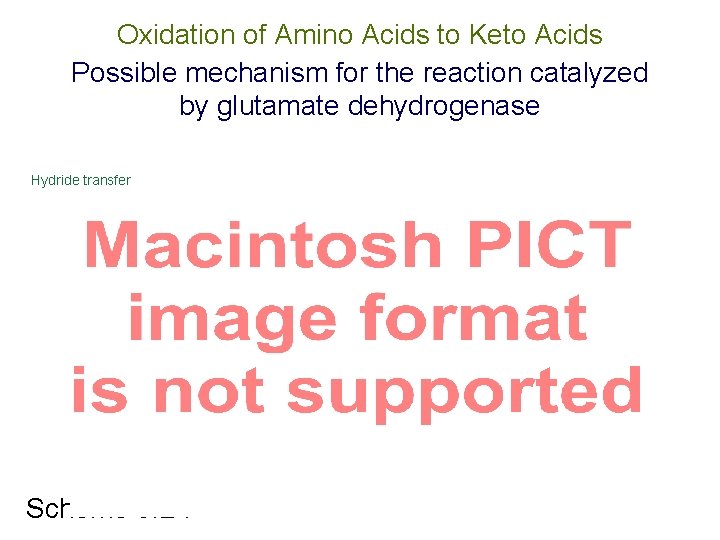
Oxidation of Amino Acids to Keto Acids Possible mechanism for the reaction catalyzed by glutamate dehydrogenase Hydride transfer Scheme 3. 24

Oxidation of Aldehydes to Carboxylic Acids (A) Covalent catalytic mechanism for the oxidation of aldehydes by aldehyde dehydrogenases; (B) noncovalent catalytic mechanism for the oxidation of aldehydes by aldehyde dehydrogenases covalent catalysis Hydride transfers Scheme 3. 25 via hydrate

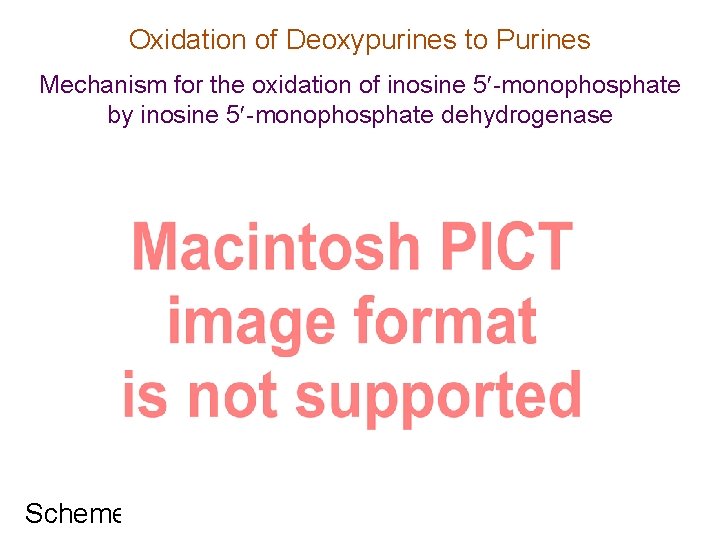
Oxidation of Deoxypurines to Purines Mechanism for the oxidation of inosine 5 -monophosphate by inosine 5 -monophosphate dehydrogenase inosine MP Scheme 3. 27 xanthine MP

An Atypical Use of NAD+ Reaction catalyzed by urocanase NAD+ in a Nonredox Reaction Scheme 3. 28
![Urocanase Reaction Run with a 13 C Pseudosubstrate substrate exchangeable proton apourocanase reconstituted with Urocanase Reaction Run with a [13 C] Pseudo-substrate “substrate” exchangeable proton apo-urocanase reconstituted with](https://slidetodoc.com/presentation_image_h2/956856d1171ecfa471fe4a5cc24c88be/image-44.jpg)
Urocanase Reaction Run with a [13 C] Pseudo-substrate “substrate” exchangeable proton apo-urocanase reconstituted with [13 C]NAD+

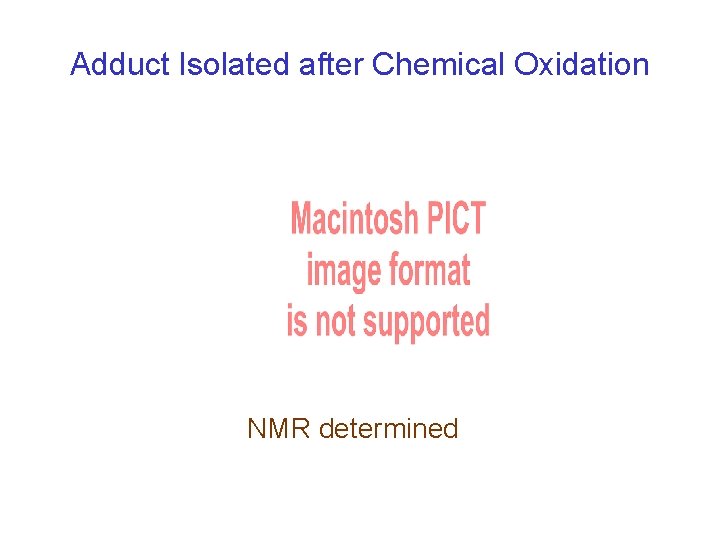
Adduct Isolated after Chemical Oxidation NMR determined

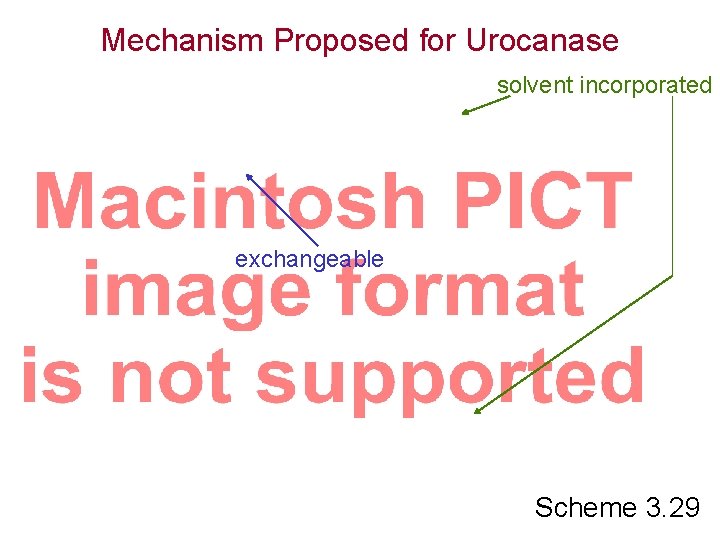
Mechanism Proposed for Urocanase solvent incorporated exchangeable Scheme 3. 29

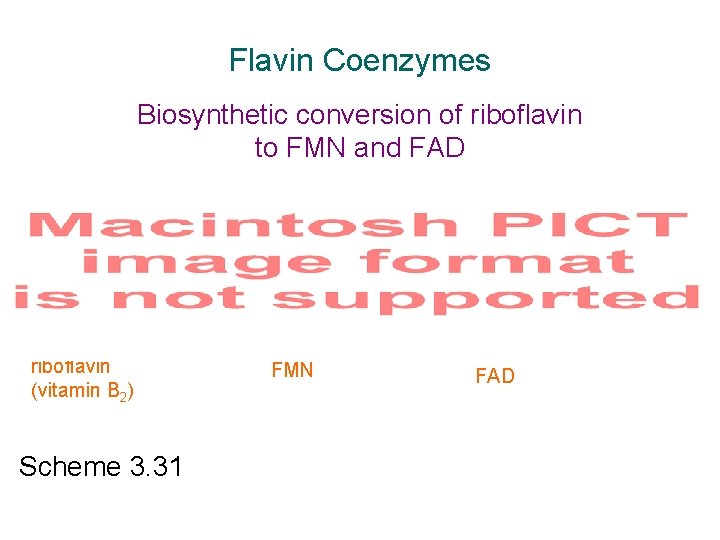
Flavin Coenzymes Biosynthetic conversion of riboflavin to FMN and FAD riboflavin (vitamin B 2) Scheme 3. 31 FMN FAD

Interconversion of the Three Oxidation States of Flavins oxidized some covalently attached to The protein at these positions Scheme 3. 32 semiquinone reduced

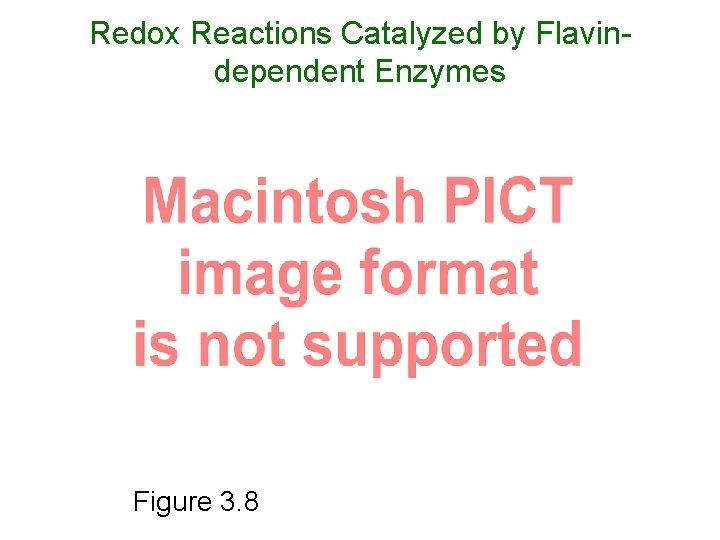
Redox Reactions Catalyzed by Flavindependent Enzymes Figure 3. 8

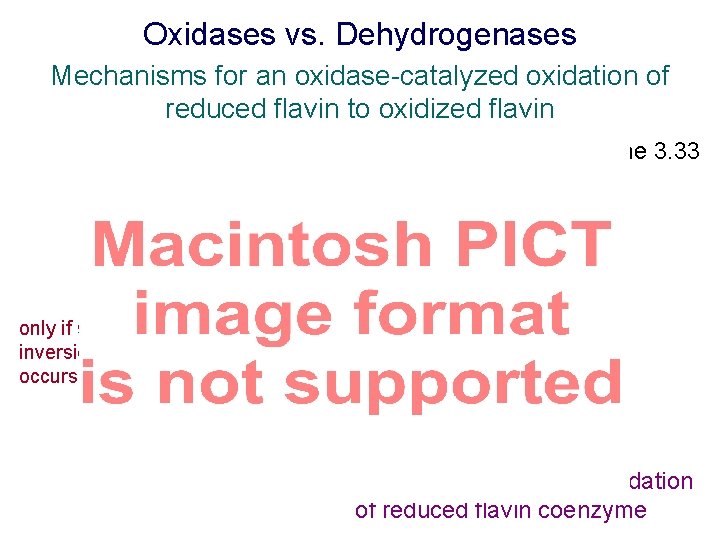
Oxidases vs. Dehydrogenases Mechanisms for an oxidase-catalyzed oxidation of reduced flavin to oxidized flavin Scheme 3. 33 only if spin inversion occurs Oxidases use O 2 for reoxidation of reduced flavin coenzyme

Dehydrogenases Use Electron Transfer Proteins to Reoxidize Reduced Flavin Mechanism for a dehydrogenase-catalyzed oxidation of reduced flavin to oxidized flavin Scheme 3. 34

Mechanisms for Flavoenzymes Overall reaction of flavoenzymes Scheme 3. 35

Mechanisms for Flavin-dependent Enzymes • Three types of mechanisms: – a carbanion intermediate – a radical intermediate – a hydride intermediate • Each of these mechanisms may be applicable to different flavoenzymes and/or different substrates

Two-Electon Mechanism (Carbanion) D-Amino acid oxidase (DAAO) catalyzes the oxidation of D-amino acids to -keto acids and ammonia

Evidence for Mechanism Ionization of substituted benzoic acids Hammett Study Derivation of the Hammett Equation Scheme 3. 36 As X becomes electron withdrawing, equilibrium constant (Ka) should increase

A Similar Relationship Should Exist for a Rate Constant (k) where Charge Develops in the Transition State Reaction of hydroxide ion with ethylsubstituted benzoates Scheme 3. 37 As X becomes electron withdrawing, rate constant (k) should increase

If Ka is measured from Scheme 3. 36 and k from Scheme 3. 37 for a series of substituents X, and the data expressed in a double logarithm plot, a straight line can be drawn

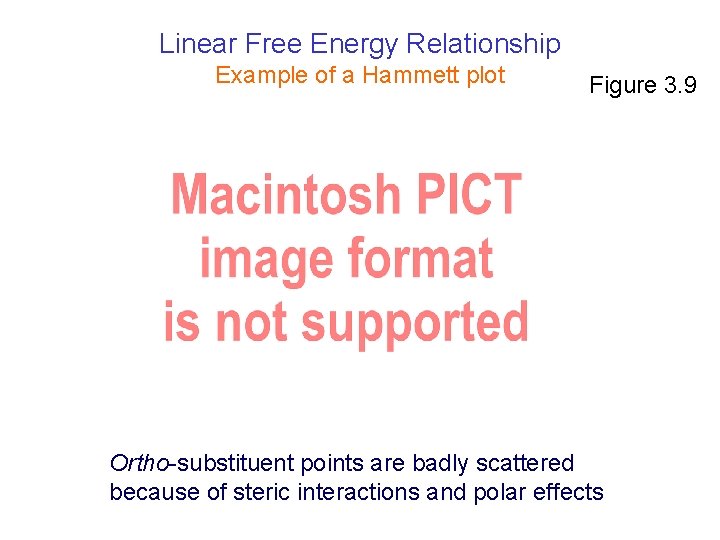
Linear Free Energy Relationship Example of a Hammett plot Figure 3. 9 Ortho-substituent points are badly scattered because of steric interactions and polar effects

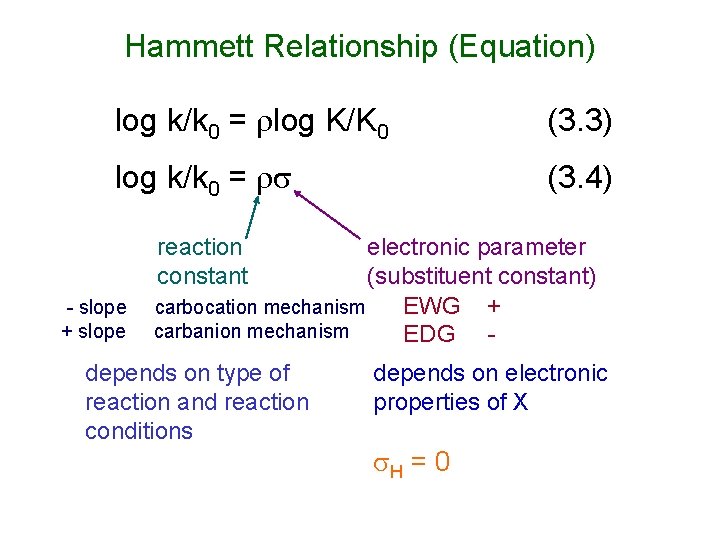
Hammett Relationship (Equation) log k/k 0 = log K/K 0 (3. 3) log k/k 0 = (3. 4) reaction constant - slope + slope electronic parameter (substituent constant) carbocation mechanism EWG + carbanion mechanism EDG - depends on type of reaction and reaction conditions depends on electronic properties of X H = 0

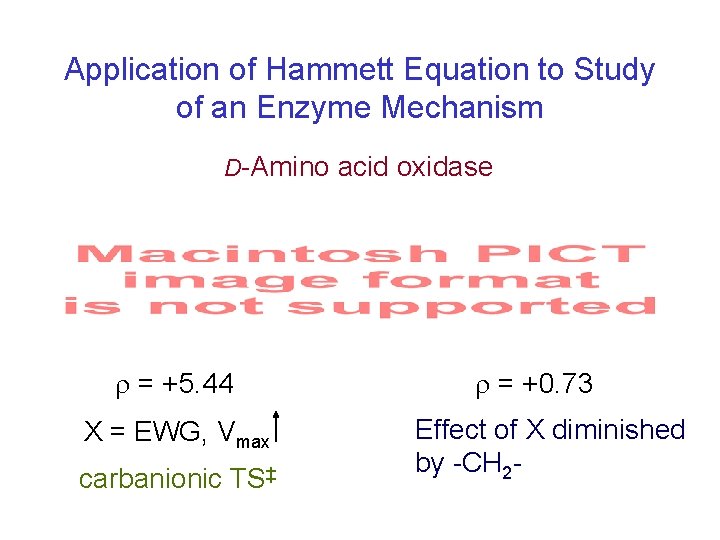
Application of Hammett Equation to Study of an Enzyme Mechanism D-Amino acid oxidase = +5. 44 X = EWG, Vmax carbanionic TS‡ = +0. 73 Effect of X diminished by -CH 2 -

Proposed Intermediate in the D-amino Acid Oxidase-catalyzed Oxidation of Substituted Phenylglycines Scheme 3. 38 What is the function of the flavin?

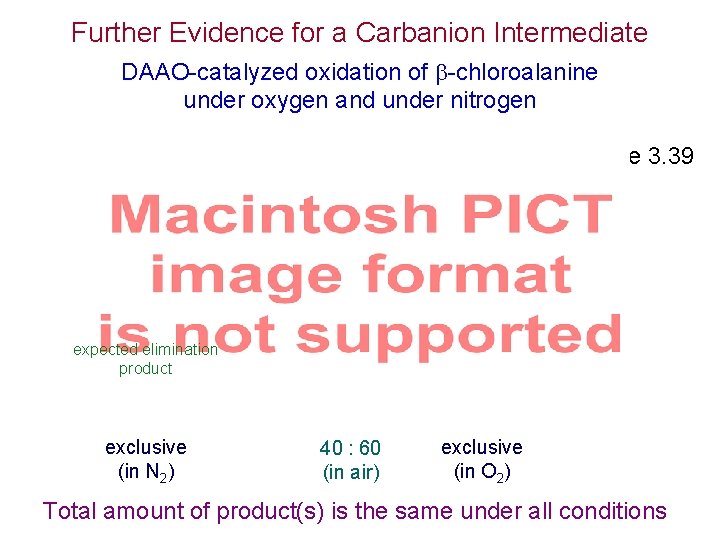
Further Evidence for a Carbanion Intermediate DAAO-catalyzed oxidation of -chloroalanine under oxygen and under nitrogen Scheme 3. 39 expected elimination product exclusive (in N 2) 40 : 60 (in air) exclusive (in O 2) Total amount of product(s) is the same under all conditions

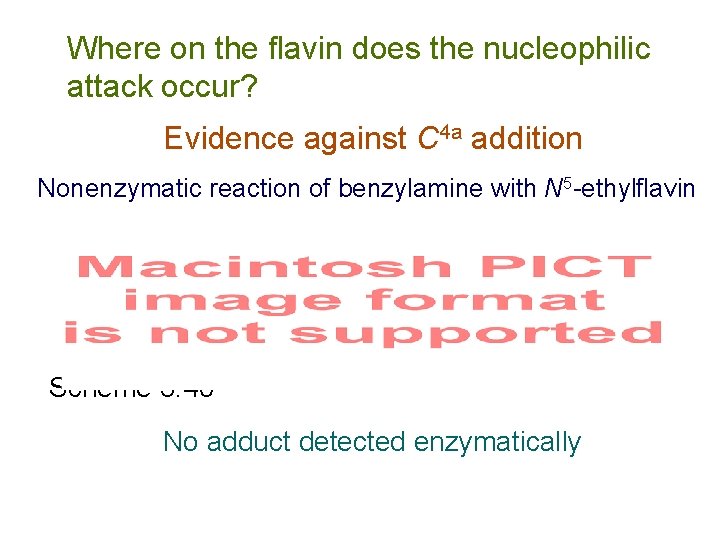
Where on the flavin does the nucleophilic attack occur? Evidence against C 4 a addition Nonenzymatic reaction of benzylamine with N 5 -ethylflavin Scheme 3. 40 No adduct detected enzymatically

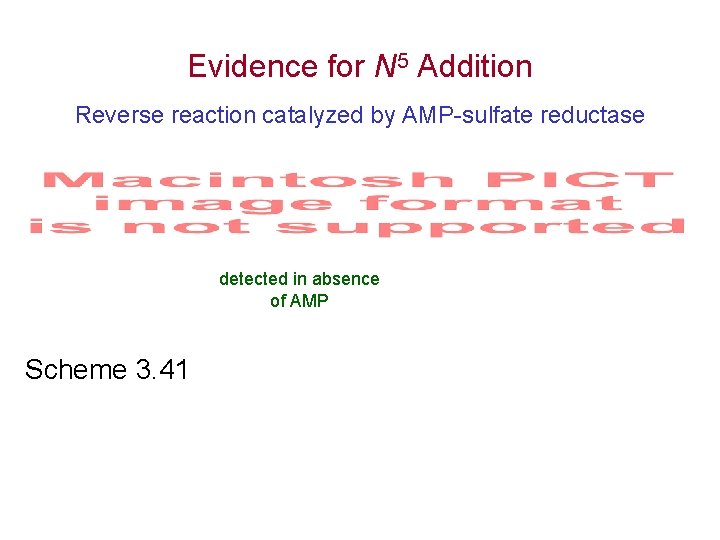
Evidence for N 5 Addition Reverse reaction catalyzed by AMP-sulfate reductase detected in absence of AMP Scheme 3. 41

Initial Evidence for N 5 Attack and for Twoelectron Chemistry NADH-dependent reduction of 5 -deazaflavin by various flavoenzymes 5 -deazaflavin Scheme 3. 42

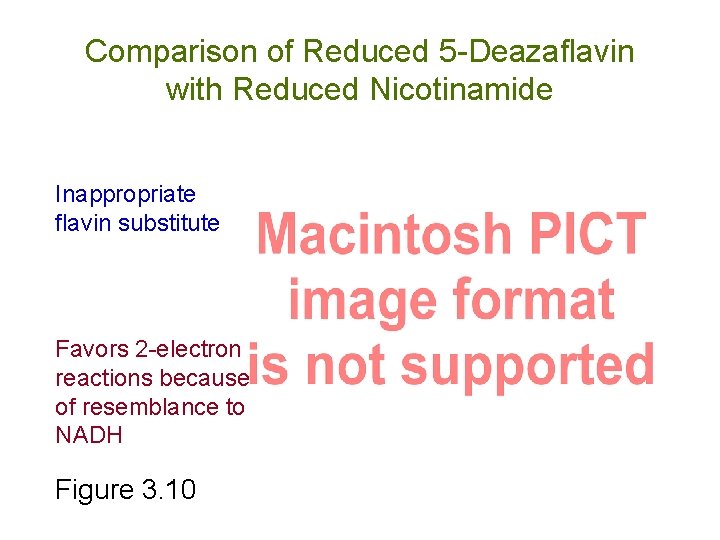
Comparison of Reduced 5 -Deazaflavin with Reduced Nicotinamide Inappropriate flavin substitute Favors 2 -electron reactions because of resemblance to NADH Figure 3. 10

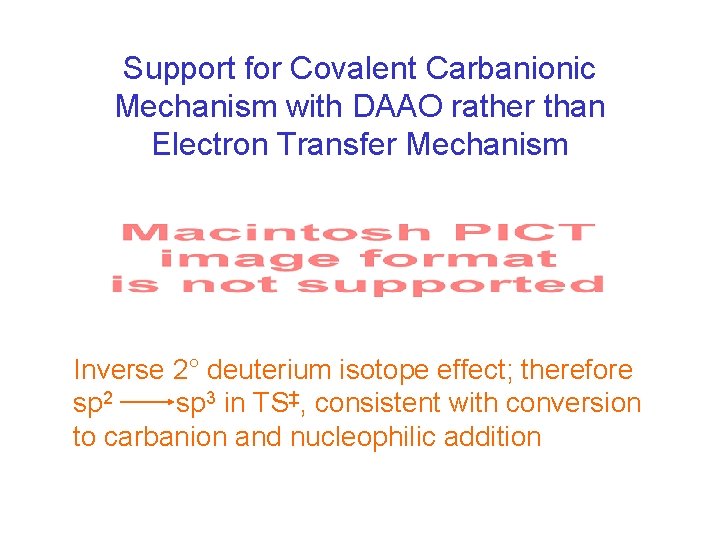
Support for Covalent Carbanionic Mechanism with DAAO rather than Electron Transfer Mechanism Inverse 2° deuterium isotope effect; therefore sp 2 sp 3 in TS‡, consistent with conversion to carbanion and nucleophilic addition

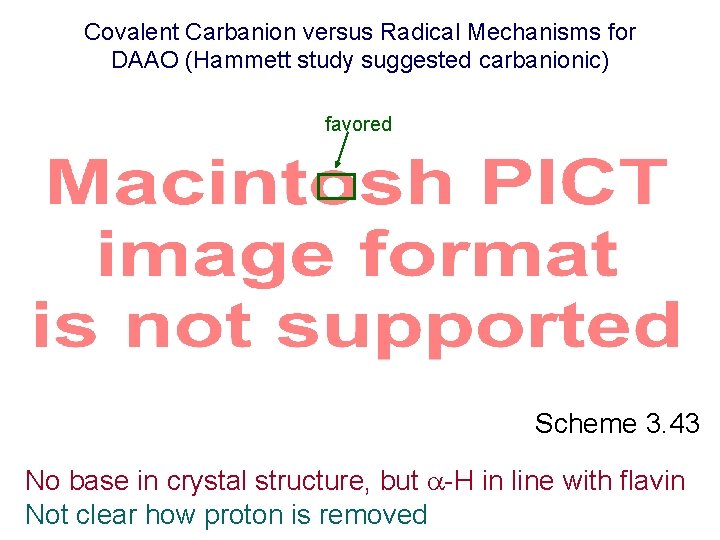
Covalent Carbanion versus Radical Mechanisms for DAAO (Hammett study suggested carbanionic) favored Scheme 3. 43 No base in crystal structure, but -H in line with flavin Not clear how proton is removed

Carbanion Mechanism Followed by 2 Oneelectron Transfers Reaction catalyzed by general acyl-Co. A dehydrogenase Scheme 3. 46

Initial Mechanism Proposed for Mechanism-based Inactivation of General Acyl-Co. A Dehydrogenase by (Methylenecyclopropyl)acetyl-Co. A Scheme 3. 47 Mechanism-based inactivator

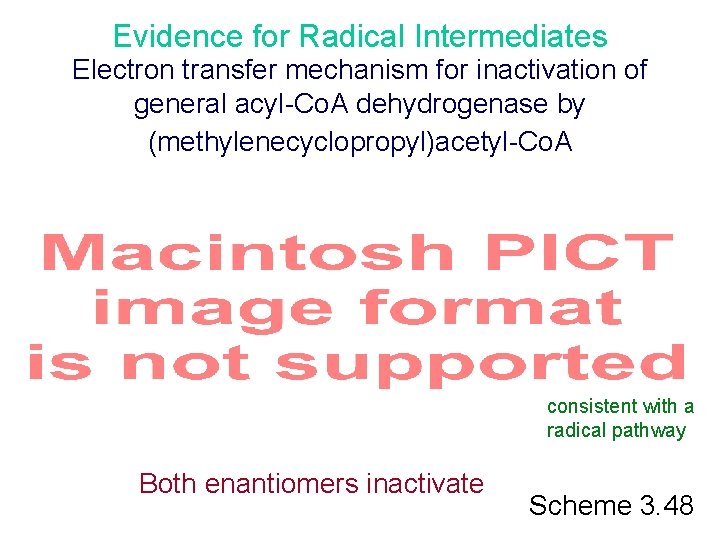
Evidence for Radical Intermediates Electron transfer mechanism for inactivation of general acyl-Co. A dehydrogenase by (methylenecyclopropyl)acetyl-Co. A only pro-R removed consistent with a radical pathway Both enantiomers inactivate Scheme 3. 48

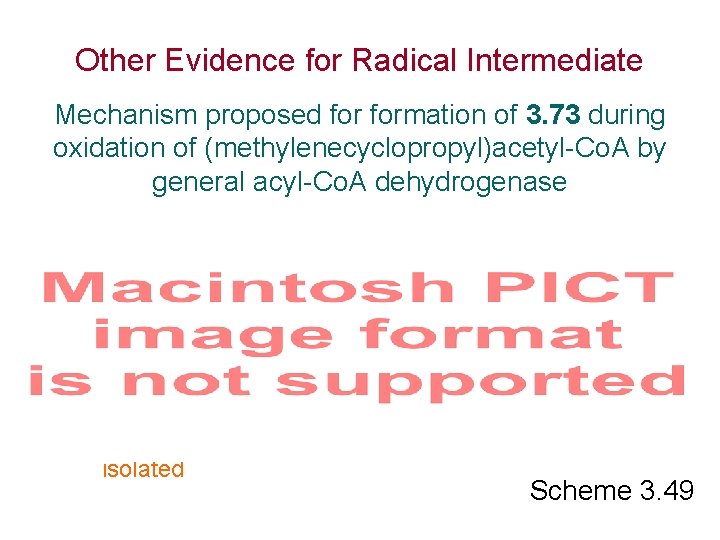
Other Evidence for Radical Intermediate Mechanism proposed formation of 3. 73 during oxidation of (methylenecyclopropyl)acetyl-Co. A by general acyl-Co. A dehydrogenase isolated Scheme 3. 49

Carbanion Followed by Single Electron Mechanism for General Acyl-Co. A Dehydrogenase Not in text

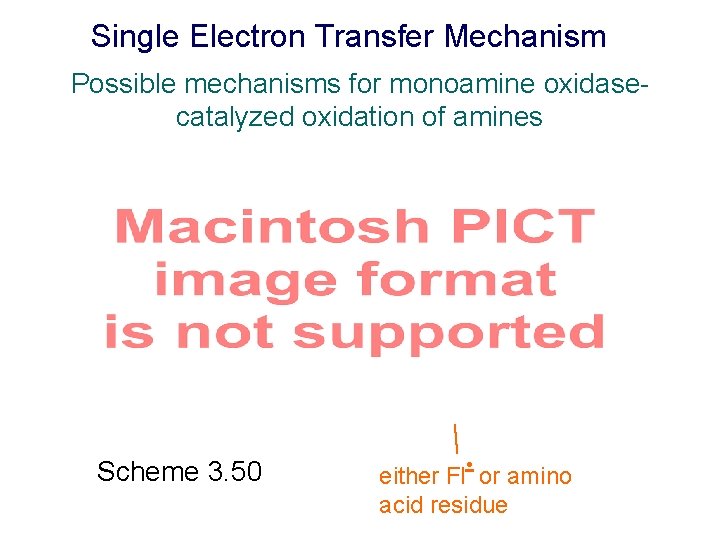
Single Electron Transfer Mechanism Possible mechanisms for monoamine oxidasecatalyzed oxidation of amines Scheme 3. 50 either Fl- • or amino acid residue

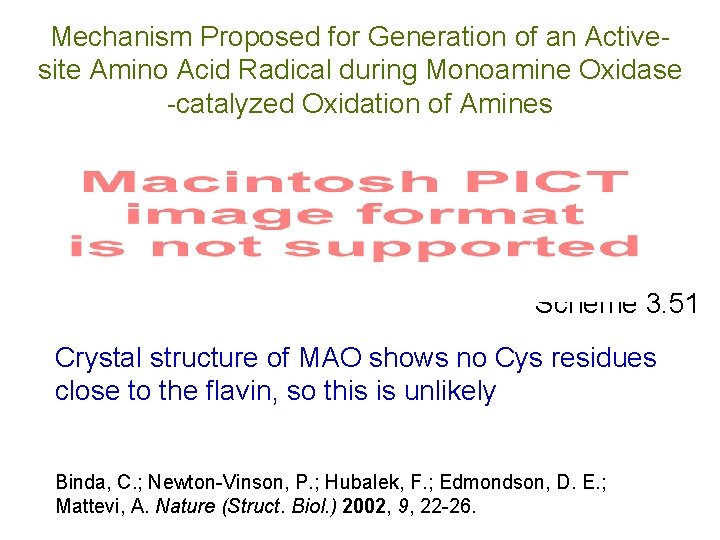
Mechanism Proposed for Generation of an Activesite Amino Acid Radical during Monoamine Oxidase -catalyzed Oxidation of Amines Scheme 3. 51 Crystal structure of MAO shows no Cys residues close to the flavin, so this is unlikely Binda, C. ; Newton-Vinson, P. ; Hubalek, F. ; Edmondson, D. E. ; Mattevi, A. Nature (Struct. Biol. ) 2002, 9, 22 -26.

Cyclopropylaminyl Radical Rearrangement Scheme 3. 52

Evidence for Aminyl Radical (radical cation? ) Mechanisms proposed for inactivation of MAO by 1 -phenylcyclopropylamine All products derived from cyclopropyl ring opening Scheme 3. 53

Chemical Reactions to Characterize the Structure of the Flavin Adduct Formed on Inactivation of MAO by 1 -Phenylcyclopropylamine Baeyer-Villiger reaction Scheme 3. 54

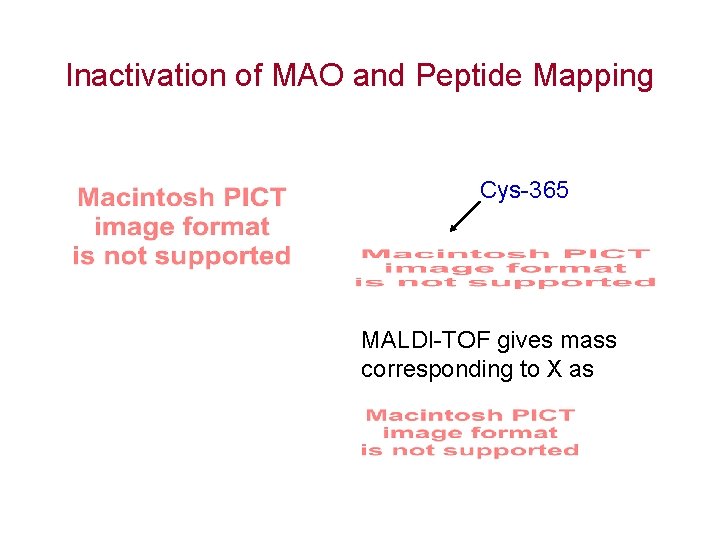
Inactivation of MAO and Peptide Mapping Cys-365 MALDI-TOF gives mass corresponding to X as

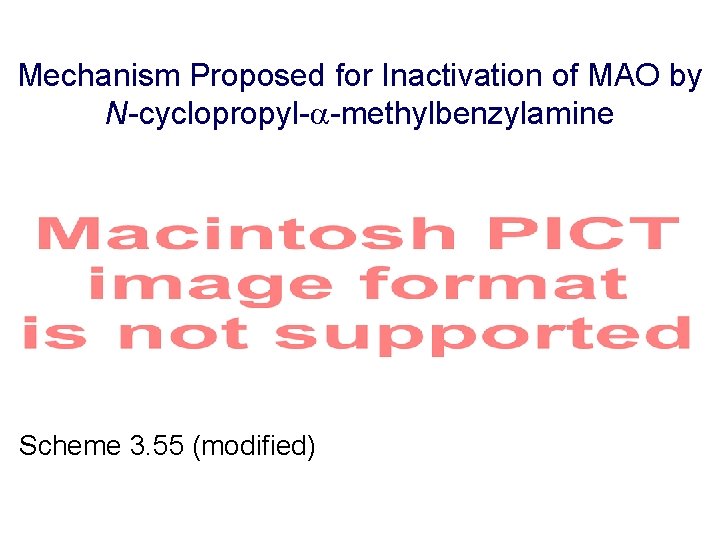
Mechanism Proposed for Inactivation of MAO by N-cyclopropyl- -methylbenzylamine Scheme 3. 55 (modified)

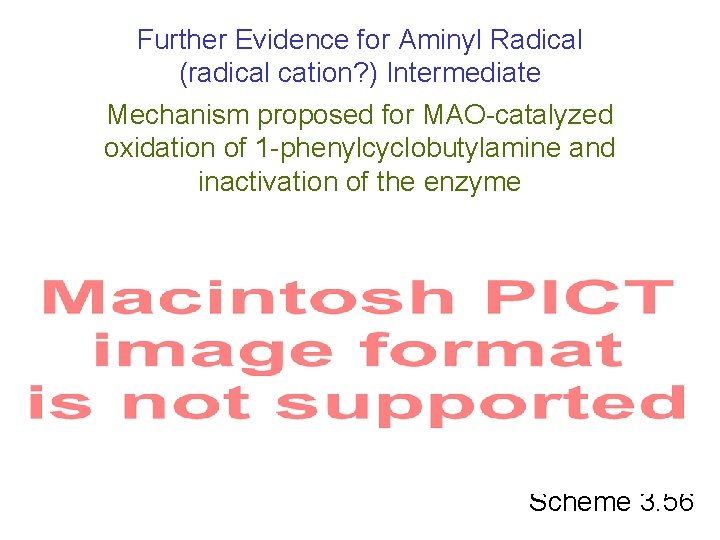
Further Evidence for Aminyl Radical (radical cation? ) Intermediate Mechanism proposed for MAO-catalyzed oxidation of 1 -phenylcyclobutylamine and inactivation of the enzyme Scheme 3. 56

Evidence for -Carbon Radical Intermediate Oxidation of (aminomethyl)cubane by MAO Gives product of a cubylcarbinyl radical intermediate detected Scheme 3. 57

Reactions to Differentiate a Radical from a Carbanion Intermediate Scheme 3. 58

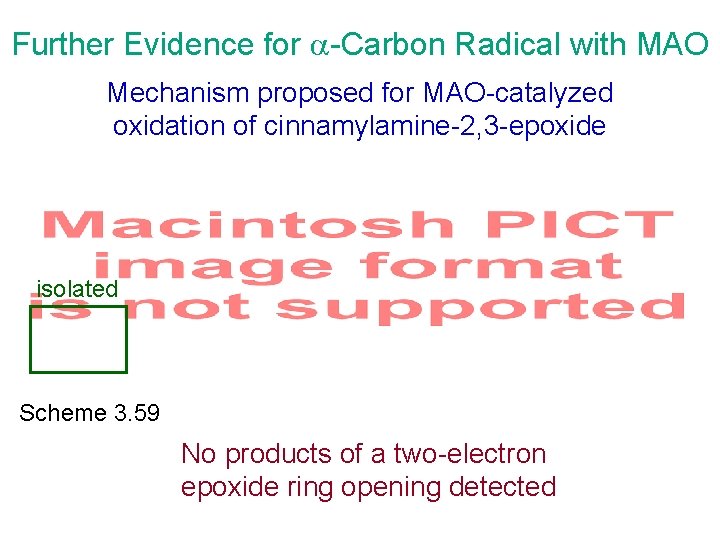
Further Evidence for -Carbon Radical with MAO Mechanism proposed for MAO-catalyzed oxidation of cinnamylamine-2, 3 -epoxide isolated Scheme 3. 59 No products of a two-electron epoxide ring opening detected

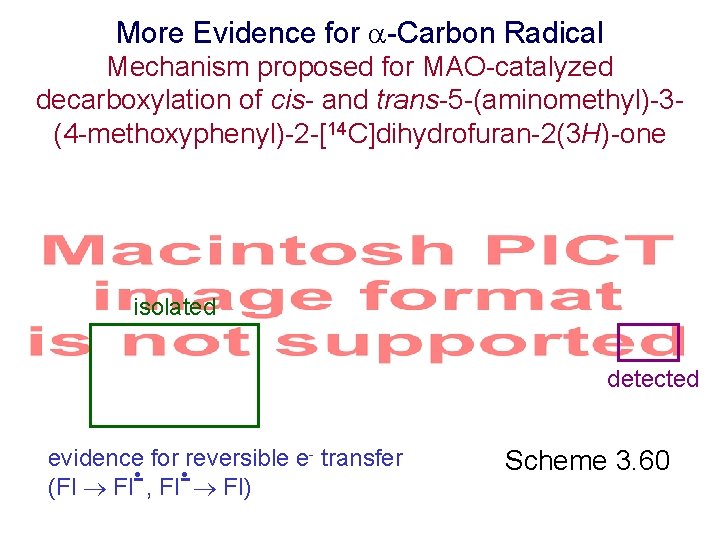
More Evidence for -Carbon Radical Mechanism proposed for MAO-catalyzed decarboxylation of cis- and trans-5 -(aminomethyl)-3(4 -methoxyphenyl)-2 -[14 C]dihydrofuran-2(3 H)-one isolated detected evidence for reversible e- transfer • • (Fl Fl- , Fl- Fl) Scheme 3. 60

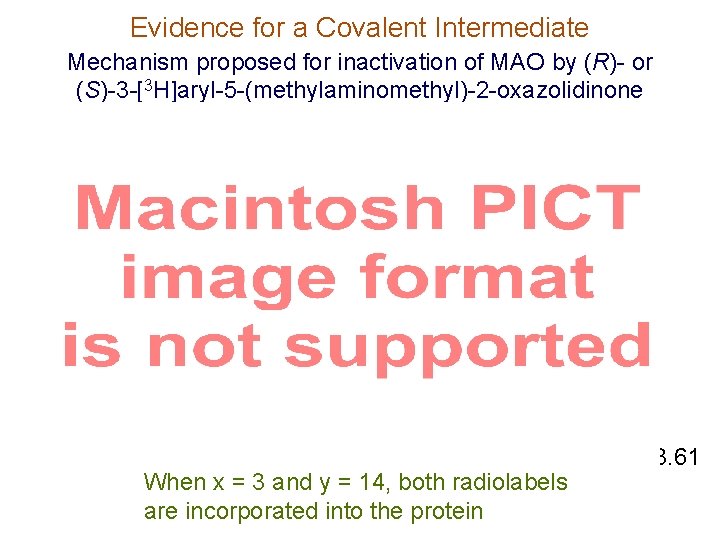
Evidence for a Covalent Intermediate Mechanism proposed for inactivation of MAO by (R)- or (S)-3 -[3 H]aryl-5 -(methylaminomethyl)-2 -oxazolidinone Scheme 3. 61 When x = 3 and y = 14, both radiolabels are incorporated into the protein

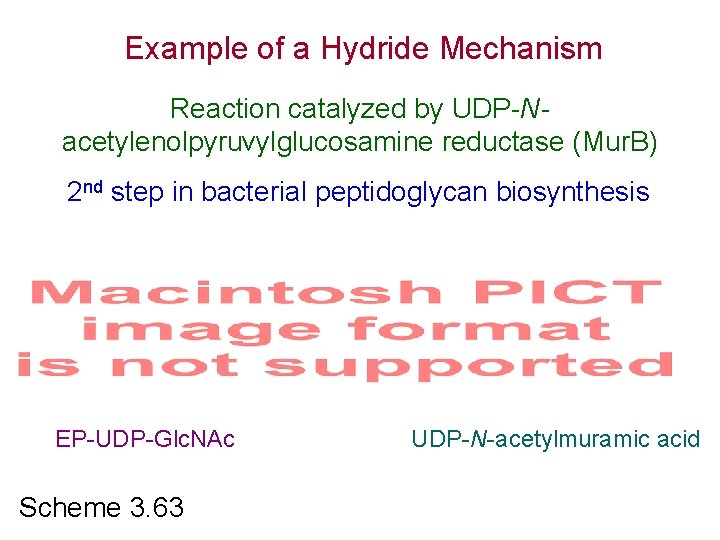
Example of a Hydride Mechanism Reaction catalyzed by UDP-Nacetylenolpyruvylglucosamine reductase (Mur. B) 2 nd step in bacterial peptidoglycan biosynthesis EP-UDP-Glc. NAc Scheme 3. 63 UDP-N-acetylmuramic acid

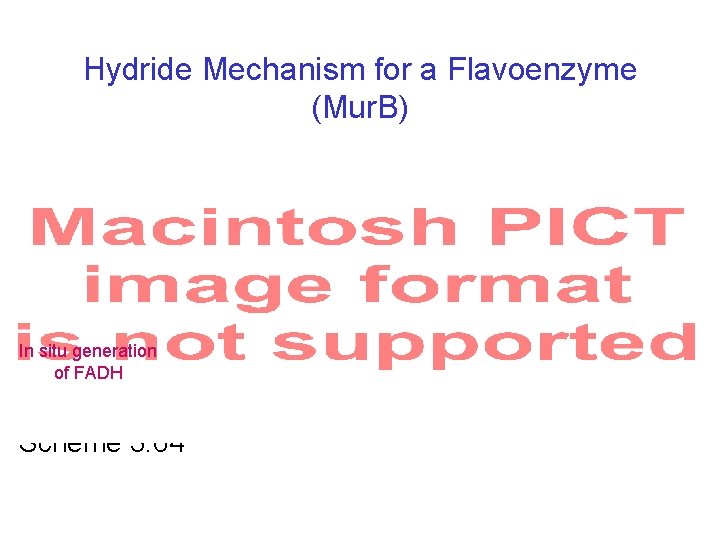
Hydride Mechanism for a Flavoenzyme (Mur. B) In situ generation of FADH Scheme 3. 64

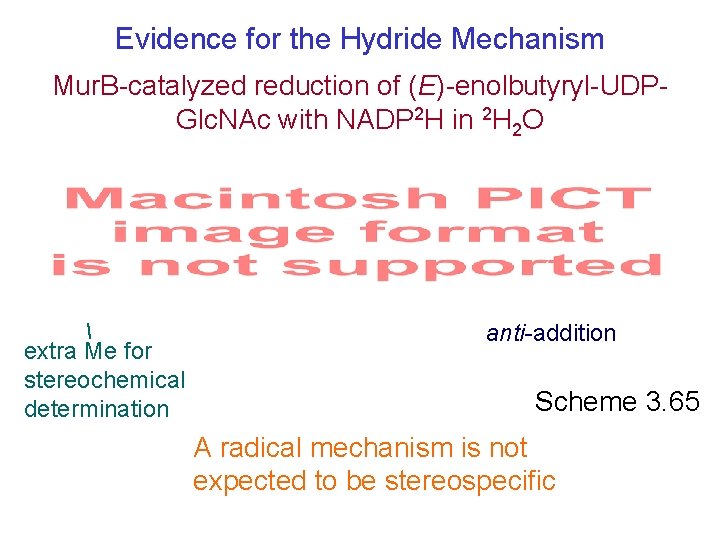
Evidence for the Hydride Mechanism Mur. B-catalyzed reduction of (E)-enolbutyryl-UDPGlc. NAc with NADP 2 H in 2 H 2 O extra Me for stereochemical determination anti-addition Scheme 3. 65 A radical mechanism is not expected to be stereospecific

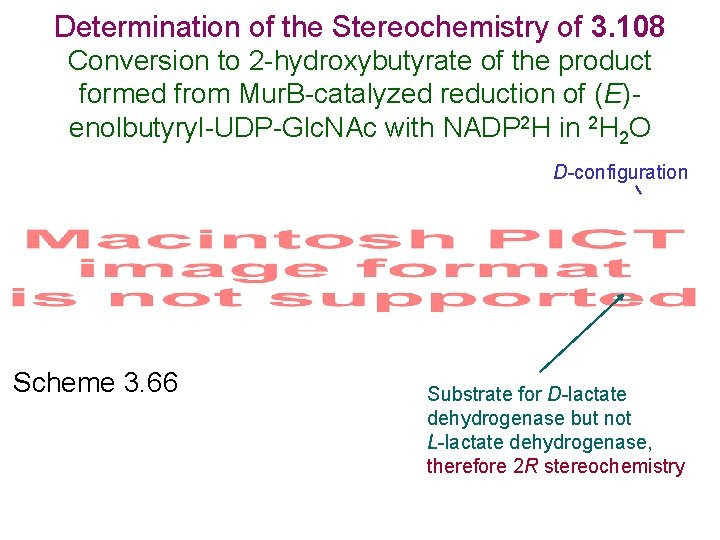
Determination of the Stereochemistry of 3. 108 Conversion to 2 -hydroxybutyrate of the product formed from Mur. B-catalyzed reduction of (E)enolbutyryl-UDP-Glc. NAc with NADP 2 H in 2 H 2 O D-configuration Scheme 3. 66 Substrate for D-lactate dehydrogenase but not L-lactate dehydrogenase, therefore 2 R stereochemistry

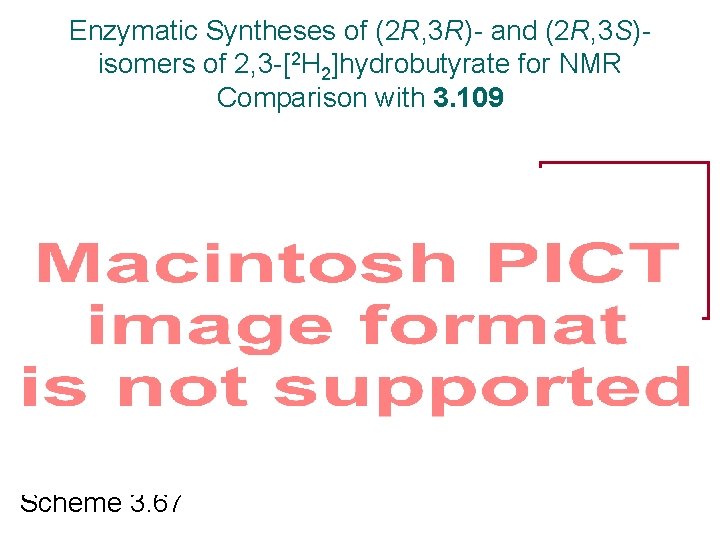
Enzymatic Syntheses of (2 R, 3 R)- and (2 R, 3 S)isomers of 2, 3 -[2 H 2]hydrobutyrate for NMR Comparison with 3. 109 omit ATP Scheme 3. 67

Stereochemistry of the Mur. B-catalyzed Reduction of (E)-enolbutyryl-UDP-Glc. NAc reface Scheme 3. 68

Reaction Catalyzed by Dihydroorotate Dehydrogenase Scheme 3. 69 D isotope effects on both H’s; therefore concerted

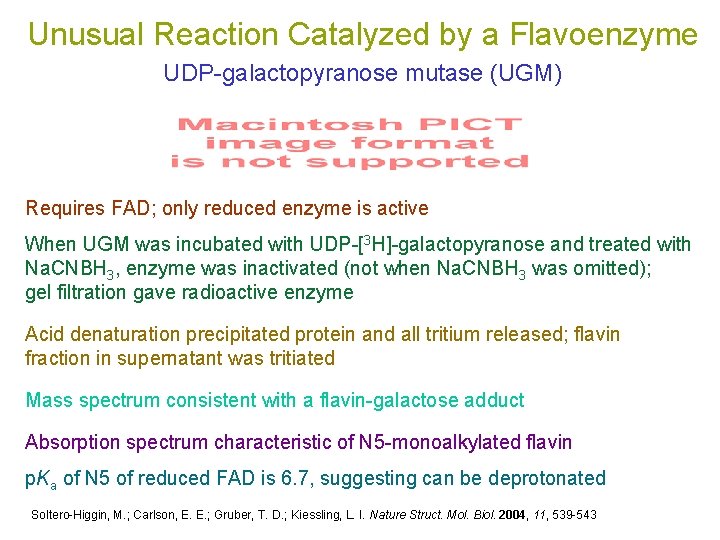
Unusual Reaction Catalyzed by a Flavoenzyme UDP-galactopyranose mutase (UGM) Requires FAD; only reduced enzyme is active When UGM was incubated with UDP-[3 H]-galactopyranose and treated with Na. CNBH 3, enzyme was inactivated (not when Na. CNBH 3 was omitted); gel filtration gave radioactive enzyme Acid denaturation precipitated protein and all tritium released; flavin fraction in supernatant was tritiated Mass spectrum consistent with a flavin-galactose adduct Absorption spectrum characteristic of N 5 -monoalkylated flavin p. Ka of N 5 of reduced FAD is 6. 7, suggesting can be deprotonated Soltero-Higgin, M. ; Carlson, E. E. ; Gruber, T. D. ; Kiessling, L. I. Nature Struct. Mol. Biol. 2004, 11, 539 -543

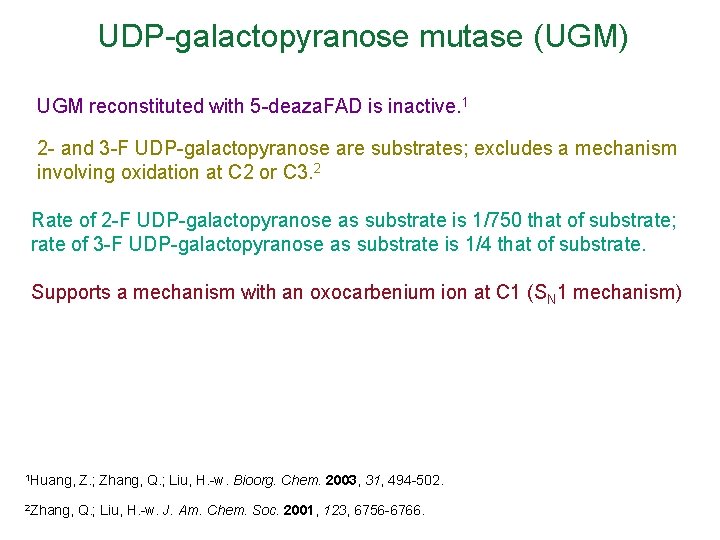
UDP-galactopyranose mutase (UGM) UGM reconstituted with 5 -deaza. FAD is inactive. 1 2 - and 3 -F UDP-galactopyranose are substrates; excludes a mechanism involving oxidation at C 2 or C 3. 2 Rate of 2 -F UDP-galactopyranose as substrate is 1/750 that of substrate; rate of 3 -F UDP-galactopyranose as substrate is 1/4 that of substrate. Supports a mechanism with an oxocarbenium ion at C 1 (SN 1 mechanism) 1 Huang, Z. ; Zhang, Q. ; Liu, H. -w. Bioorg. Chem. 2003, 31, 494 -502. 2 Zhang, Q. ; Liu, H. -w. J. Am. Chem. Soc. 2001, 123, 6756 -6766.

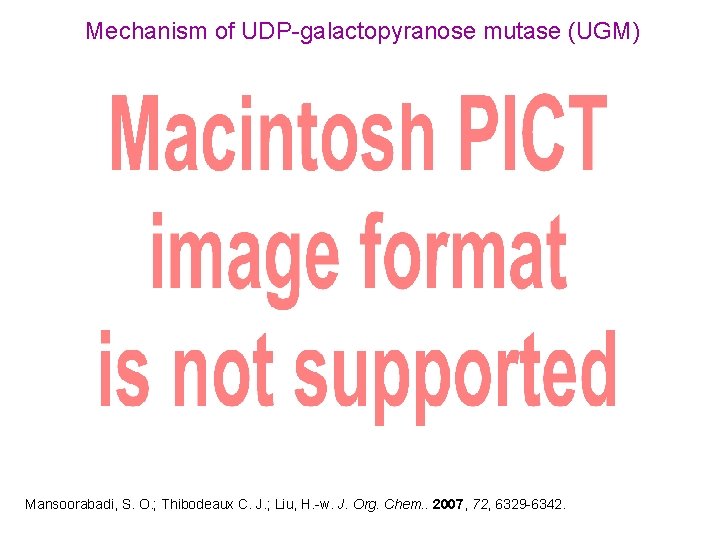
Mechanism of UDP-galactopyranose mutase (UGM) Mansoorabadi, S. O. ; Thibodeaux C. J. ; Liu, H. -w. J. Org. Chem. . 2007, 72, 6329 -6342.

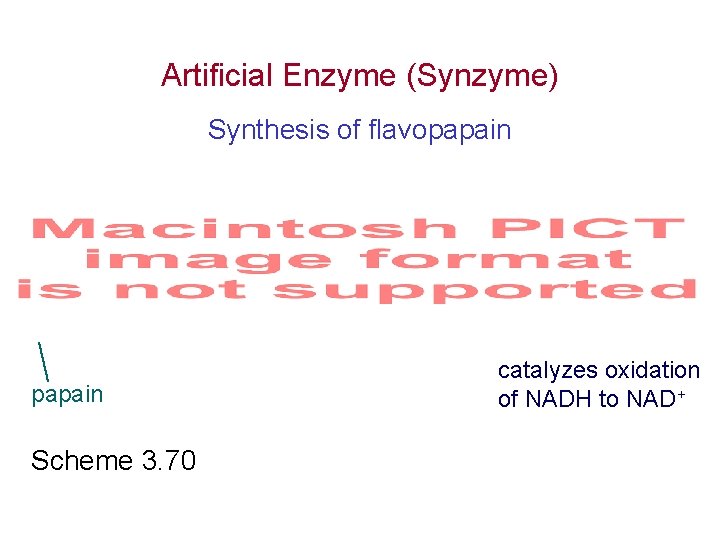
Artificial Enzyme (Synzyme) Synthesis of flavopapain Scheme 3. 70 catalyzes oxidation of NADH to NAD+

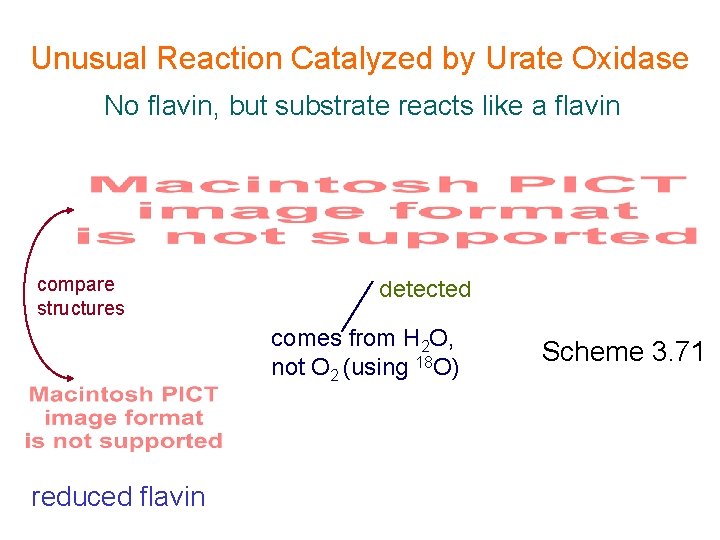
Unusual Reaction Catalyzed by Urate Oxidase No flavin, but substrate reacts like a flavin compare structures detected comes from H 2 O, not O 2 (using 18 O) reduced flavin Scheme 3. 71

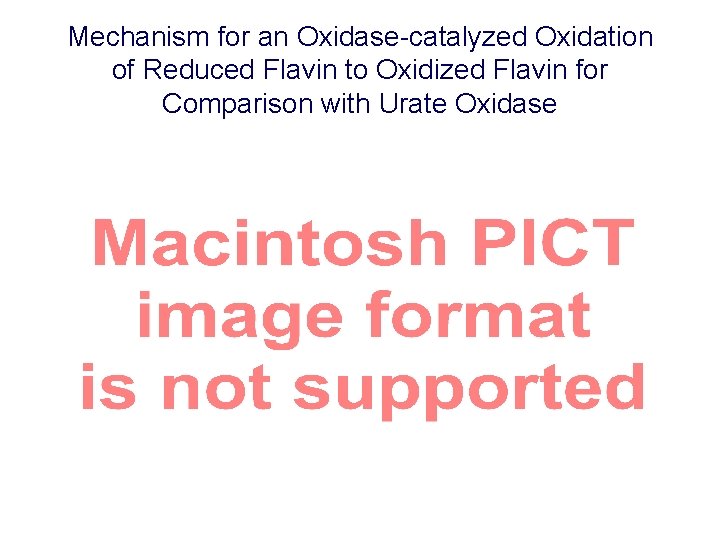
Mechanism for an Oxidase-catalyzed Oxidation of Reduced Flavin to Oxidized Flavin for Comparison with Urate Oxidase Scheme 3. 33

Possible Mechanism for the Urate Oxidase-catalyzed Oxidation of Urate detected Scheme 3. 72 Just like mechanism for oxidation of reduced flavin by O 2

Pyrroloquinoline Quinone Coenzymes (PQQ) Bound to quinoproteins Also called methoxatin, coenzyme PQQ

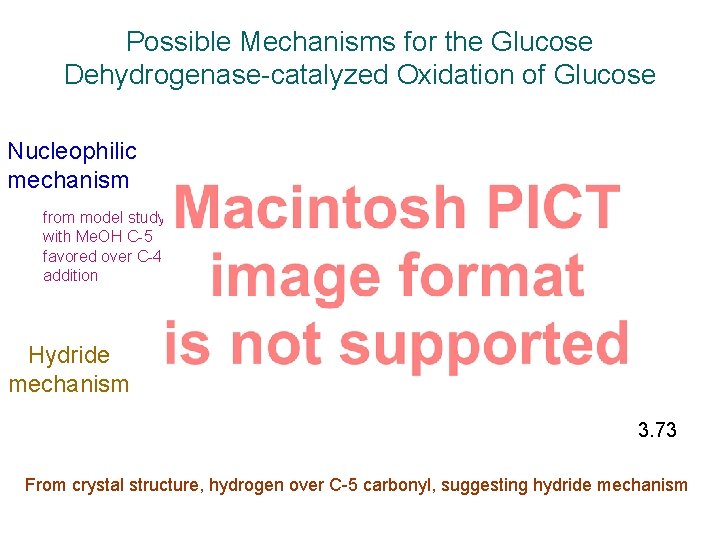
Possible Mechanisms for the Glucose Dehydrogenase-catalyzed Oxidation of Glucose Nucleophilic mechanism from model study with Me. OH C-5 favored over C-4 addition Hydride mechanism Scheme 3. 73 From crystal structure, hydrogen over C-5 carbonyl, suggesting hydride mechanism

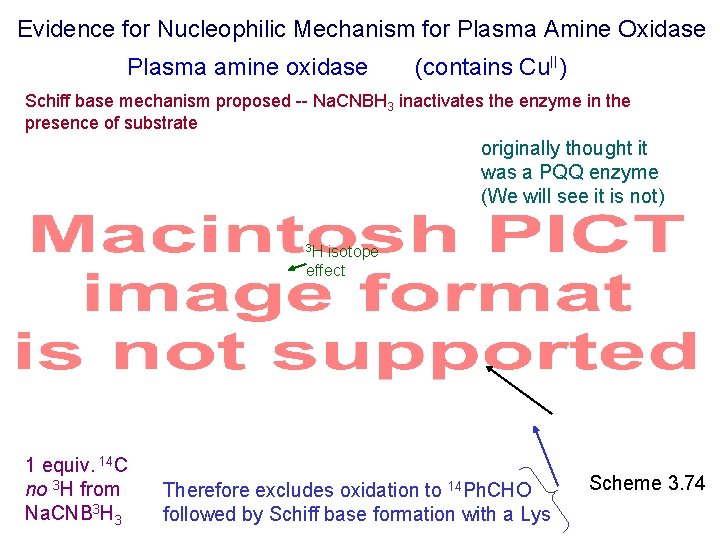
Evidence for Nucleophilic Mechanism for Plasma Amine Oxidase Plasma amine oxidase (contains Cu. II) Schiff base mechanism proposed -- Na. CNBH 3 inactivates the enzyme in the presence of substrate originally thought it was a PQQ enzyme (We will see it is not) 3 H isotope effect 1 equiv. 14 C no 3 H from Na. CNB 3 H 3 Therefore excludes oxidation to 14 Ph. CHO followed by Schiff base formation with a Lys Scheme 3. 74

Isotope Labeling Shows Syn Hydrogens are Removed (one-base mechanism) Stereochemistry of the reaction catalyzed by plasma amine oxidase (PAO) PQQ is not the actual cofactor for PAO Scheme 3. 75

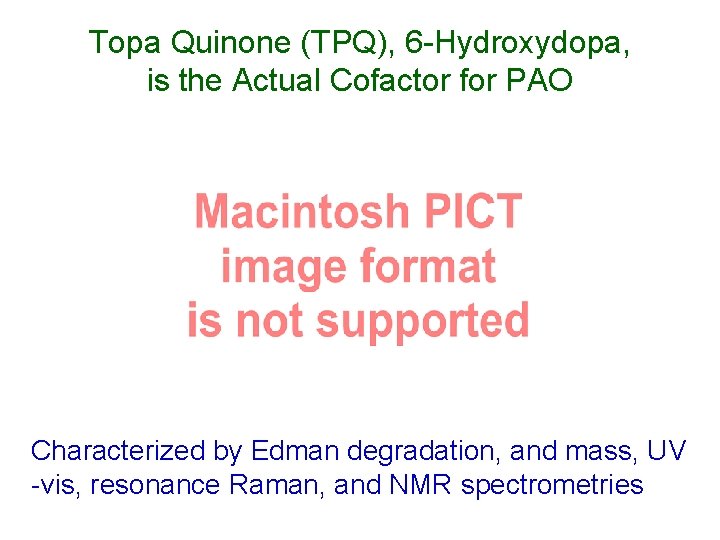
Topa Quinone (TPQ), 6 -Hydroxydopa, is the Actual Cofactor for PAO Characterized by Edman degradation, and mass, UV -vis, resonance Raman, and NMR spectrometries

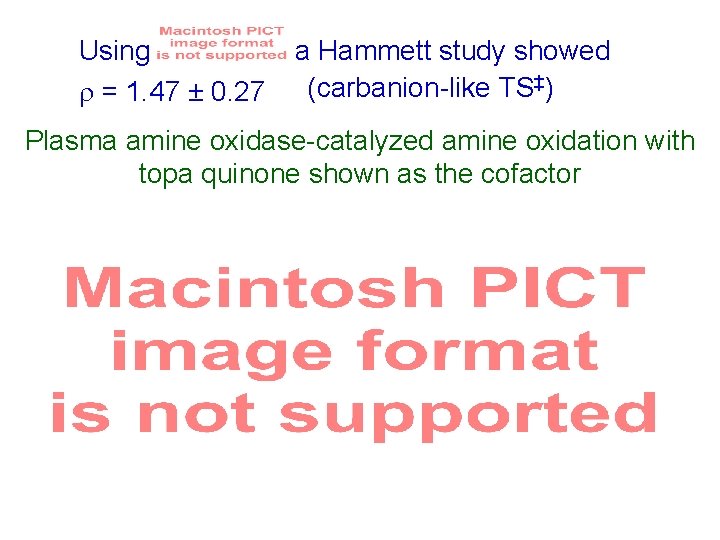
Using = 1. 47 ± 0. 27 a Hammett study showed (carbanion-like TS‡) Plasma amine oxidase-catalyzed amine oxidation with topa quinone shown as the cofactor Scheme 3. 76

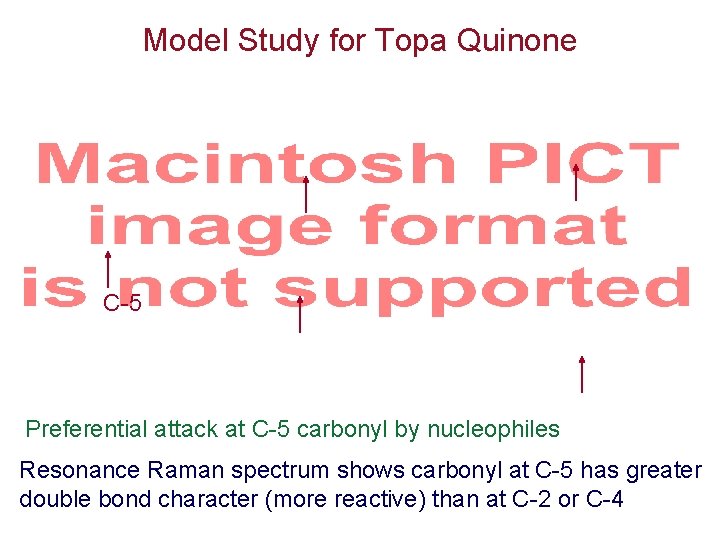
Model Study for Topa Quinone C-5 Preferential attack at C-5 carbonyl by nucleophiles Resonance Raman spectrum shows carbonyl at C-5 has greater double bond character (more reactive) than at C-2 or C-4

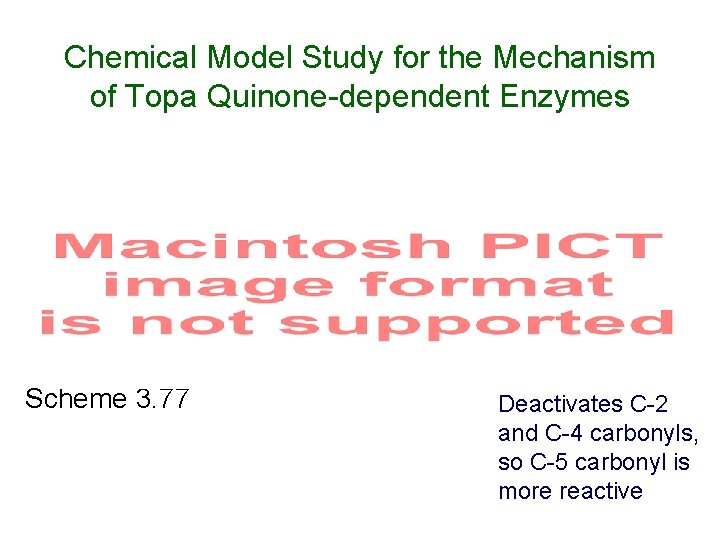
Chemical Model Study for the Mechanism of Topa Quinone-dependent Enzymes Scheme 3. 77 Deactivates C-2 and C-4 carbonyls, so C-5 carbonyl is more reactive

Detailed Mechanism Proposed for Topa Quinone-dependent Enzymes Mechanism for Plasma Amine Oxidase Scheme 3. 79

Mechanism Proposed for Reoxidation of Reduced Topa Quinone Based on EPR spectroscopy Scheme 3. 80 detected

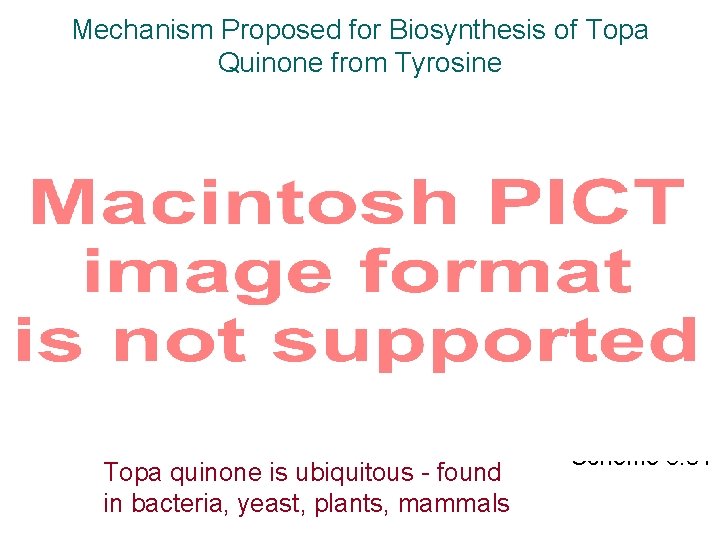
Mechanism Proposed for Biosynthesis of Topa Quinone from Tyrosine Topa quinone is ubiquitous - found in bacteria, yeast, plants, mammals Scheme 3. 81

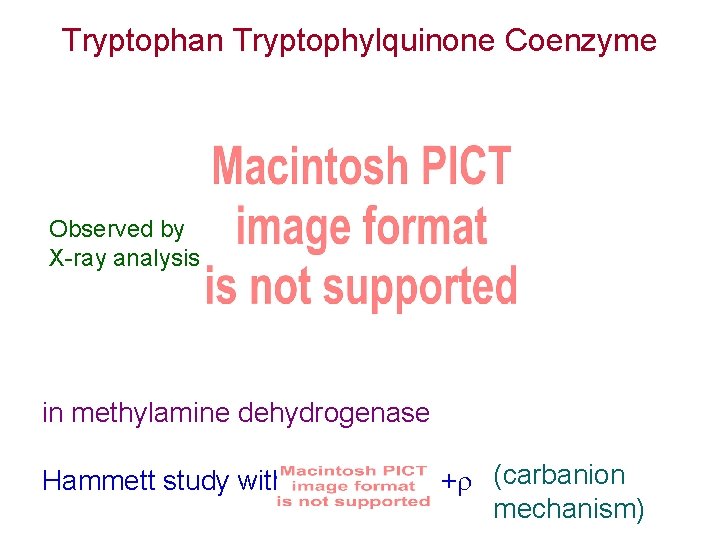
Tryptophan Tryptophylquinone Coenzyme Observed by X-ray analysis in methylamine dehydrogenase Hammett study with + (carbanion mechanism)

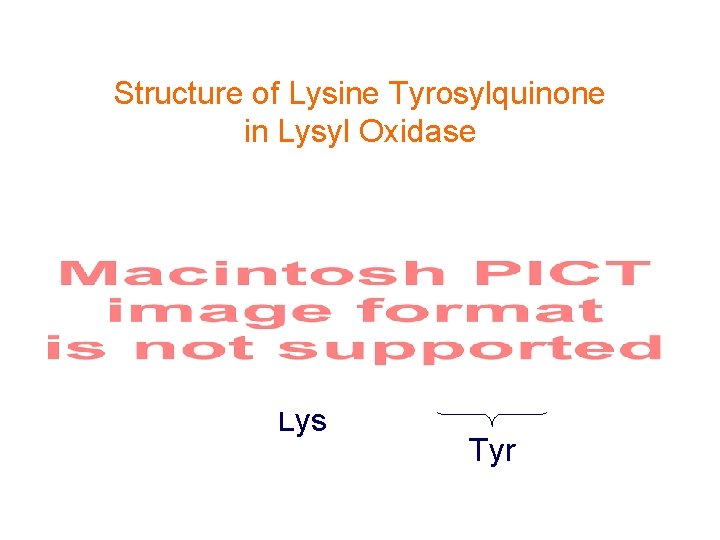
Coenzyme in Lysyl Oxidase Isolated from a proteolytic digestion

Structure of Lysine Tyrosylquinone in Lysyl Oxidase Lys Tyr

Enzymes Containing Amino Acid Radicals Mechanism proposed for galactose oxidase using a covalently bonded cysteine cross-linked tyrosine radical Scheme 3. 82

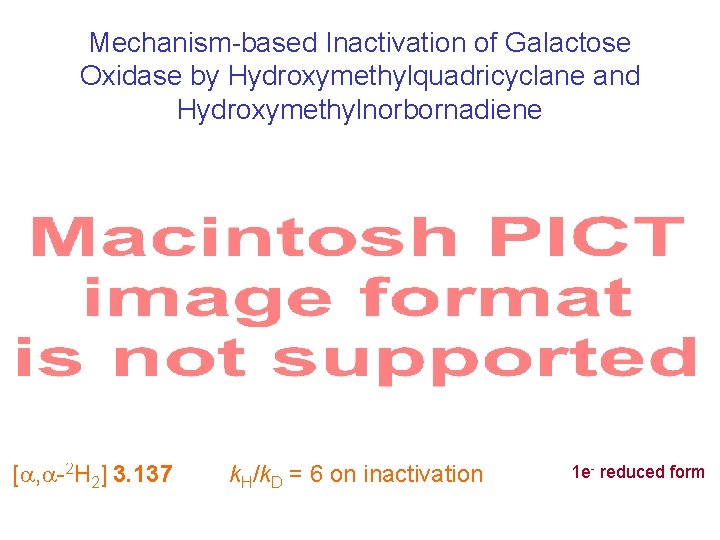
Mechanism-based Inactivation of Galactose Oxidase by Hydroxymethylquadricyclane and Hydroxymethylnorbornadiene Scheme 3. 83 quadricyclane analogue ketyl radicals norbornadiene analogue [ , -2 H 2] 3. 137 k. H/k. D = 6 on inactivation 1 e- reduced form

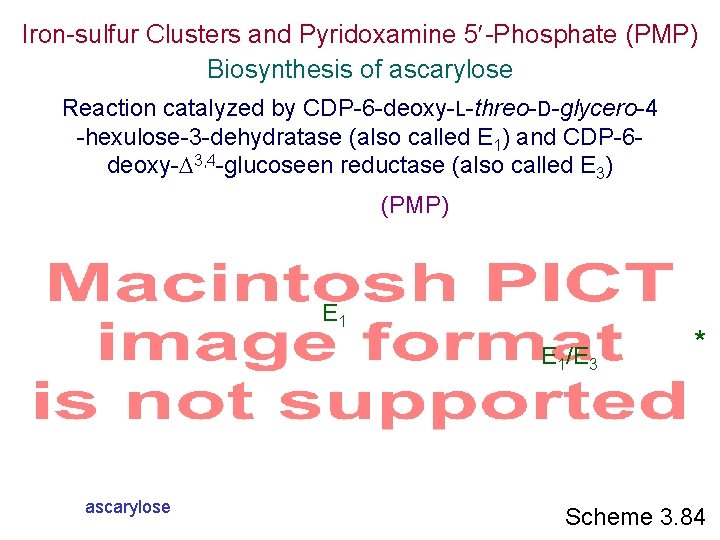
Iron-sulfur Clusters and Pyridoxamine 5 -Phosphate (PMP) Biosynthesis of ascarylose Reaction catalyzed by CDP-6 -deoxy-L-threo-D-glycero-4 -hexulose-3 -dehydratase (also called E 1) and CDP-6 deoxy- 3, 4 -glucoseen reductase (also called E 3) (PMP) E 1/E 3 ascarylose * Scheme 3. 84

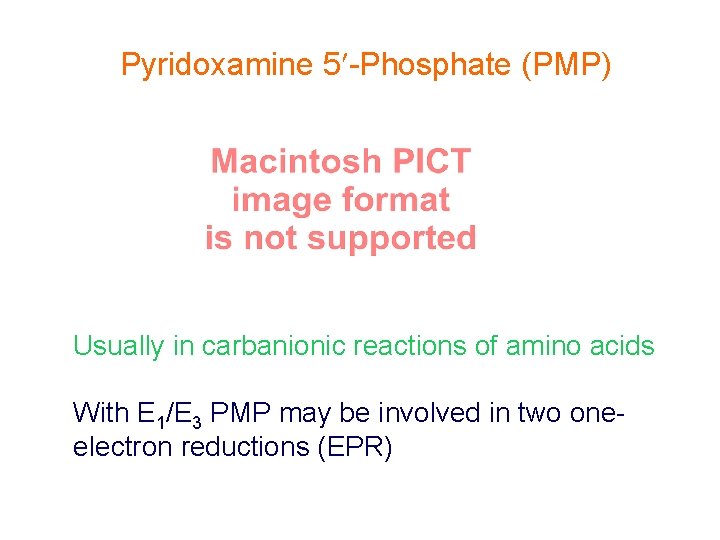
Pyridoxamine 5 -Phosphate (PMP) Usually in carbanionic reactions of amino acids With E 1/E 3 PMP may be involved in two oneelectron reductions (EPR)
![Ironsulfur Clusters 2 Fe2 S 3 Fe4 S 4 Fe4 S 1 electron and Iron-sulfur Clusters [2 Fe-2 S] [3 Fe-4 S] [4 Fe-4 S] 1 electron and](https://slidetodoc.com/presentation_image_h2/956856d1171ecfa471fe4a5cc24c88be/image-119.jpg)
Iron-sulfur Clusters [2 Fe-2 S] [3 Fe-4 S] [4 Fe-4 S] 1 electron and 2 electron transfers

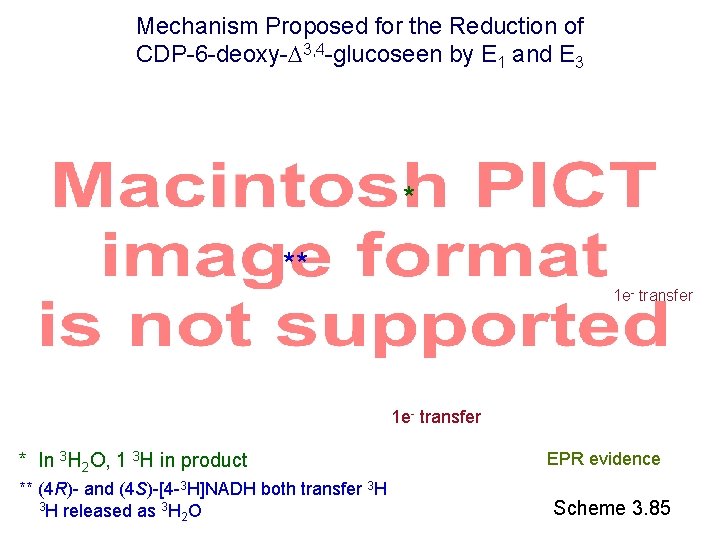
Mechanism Proposed for the Reduction of CDP-6 -deoxy- 3, 4 -glucoseen by E 1 and E 3 * ** 1 e- transfer * In 3 H 2 O, 1 3 H in product ** (4 R)- and (4 S)-[4 -3 H]NADH both transfer 3 H 3 H released as 3 H O 2 EPR evidence Scheme 3. 85

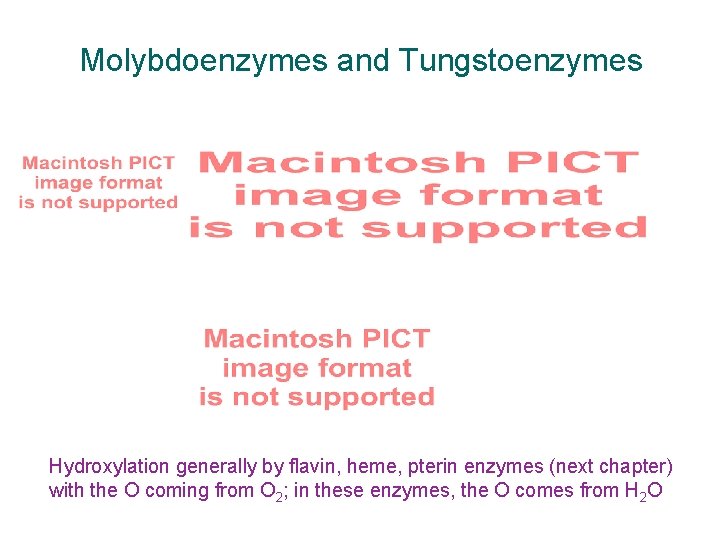
Molybdoenzymes and Tungstoenzymes Hydroxylation generally by flavin, heme, pterin enzymes (next chapter) with the O coming from O 2; in these enzymes, the O comes from H 2 O

Mechanism for Sulfite Oxidase (in liver) O from H 2 O Scheme 3. 86

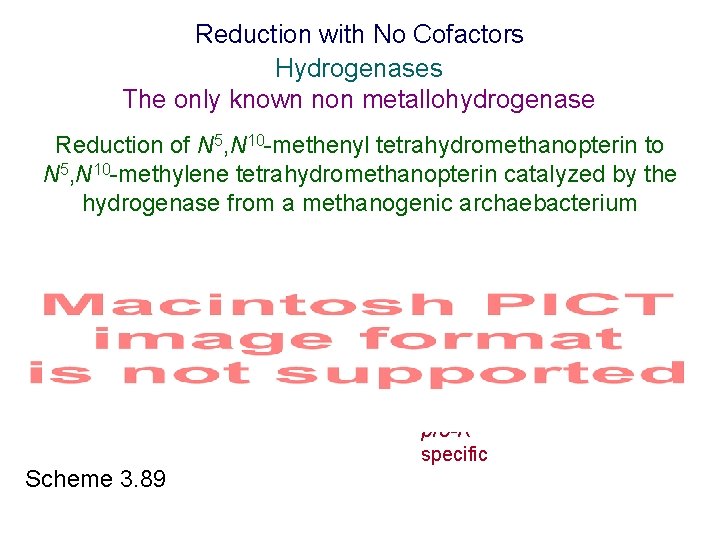
Reduction with No Cofactors Hydrogenases The only known non metallohydrogenase Reduction of N 5, N 10 -methenyl tetrahydromethanopterin to N 5, N 10 -methylene tetrahydromethanopterin catalyzed by the hydrogenase from a methanogenic archaebacterium Scheme 3. 89 pro-R specific

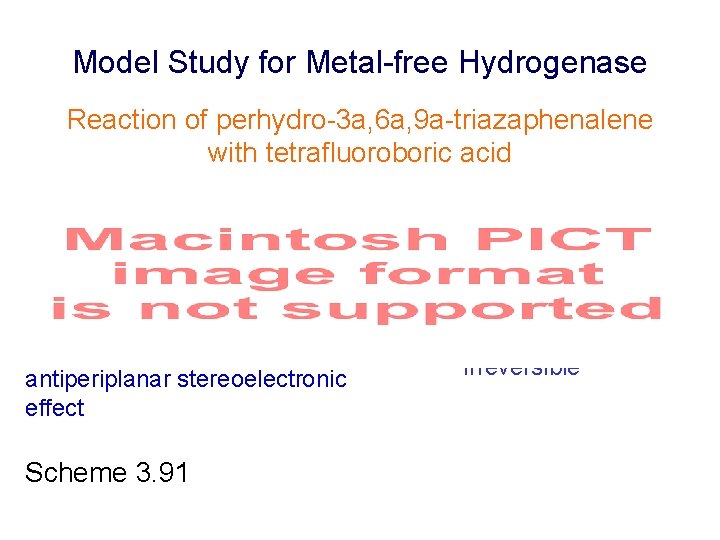
Model Study for Metal-free Hydrogenase Reaction of perhydro-3 a, 6 a, 9 a-triazaphenalene with tetrafluoroboric acid 110 °C strong acid antiperiplanar stereoelectronic effect Scheme 3. 91 irreversible

Mechanism Proposed for Oxidation of N 5, N 10 -methylene tetrahydromethanopterin to N 5, N 10 -methenyl tetrahydromethanopterin (reverse of the reaction in Scheme 3. 89) initially, not resonance stabilized Scheme 3. 90 conformational change