The Oncotype DX Breast Cancer Assay 21 gene

The Oncotype DX® Breast Cancer Assay 21 -gene assay individualising treatment options 1
Agenda 2

Genomic Health • • Founded in 2000 Based in Redwood City, California, USA European headquarters in Geneva, Switzerland Oncotype DX® assays • • Breast (invasive) DCIS Prostate Colon The world-leading provider of genomic-based diagnostic assays 3
Oncotype DX® Breast Cancer Assay • Intended for newly diagnosed patients with early-stage ER+, HER 2 -, invasive breast cancer, helping to make the best possible treatment decision for every patient. • The ONLY multi-gene assay that quantifies risk of disease recurrence and likelihood of benefit from chemotherapy. • The ONLY assay consistently incorporated into the 5 internationally recognized clinical guidelines (ASCO, NCCN, St. Gallen, ESMO, and NICE ) based on strong clinical evidence, a high level of standardisation and cost effectiveness. • A proven 10 year track record with over 450, 000 patients with breast cancer tested worldwide. 4

Two Important Questions Asked by Patients Will my cancer come back? Do I need chemotherapy? 5
Traditional Parameters Used to Inform Treatment Decisions Current markers are prognostic, not predictive, of chemotherapy benefit • Tumour size • Grade • Patient age • Lymph node status • ER / PR, HER 2, Ki-671 6 1 Viale G et al. JNCI 2008; 100: 207 -212
Population-Based Tools Used to Estimate Treatment Benefit Current tools are prognostic, not predictive, of chemotherapy benefit • Adjuvant! Online 1, 2 • Nottingham Prognostic Index 1, 3 • Predict 4 7 1 NICE Diagnostics Guidance 10, 2013; 2 Balakrishnan A et al Ann Oncol 2011; 22: 1461 -2; 3 Haybittle JL et al Br J Cancer 1982; 45: 361 -366; 4 Wishart GC et al Breast Cancer Res 2010; 12: R 1
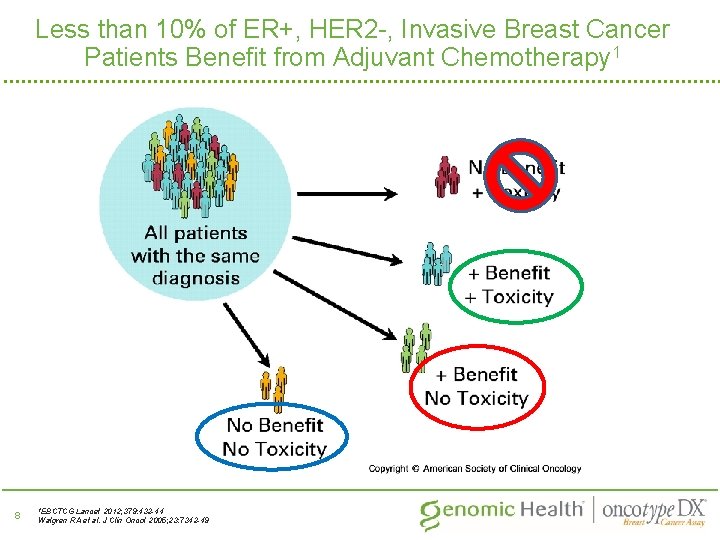
Less than 10% of ER+, HER 2 -, Invasive Breast Cancer Patients Benefit from Adjuvant Chemotherapy 1 8 1 EBCTCG Lancet 2012; 379: 432 -44 Walgren RA et al. J Clin Oncol 2005; 23: 7342 -49
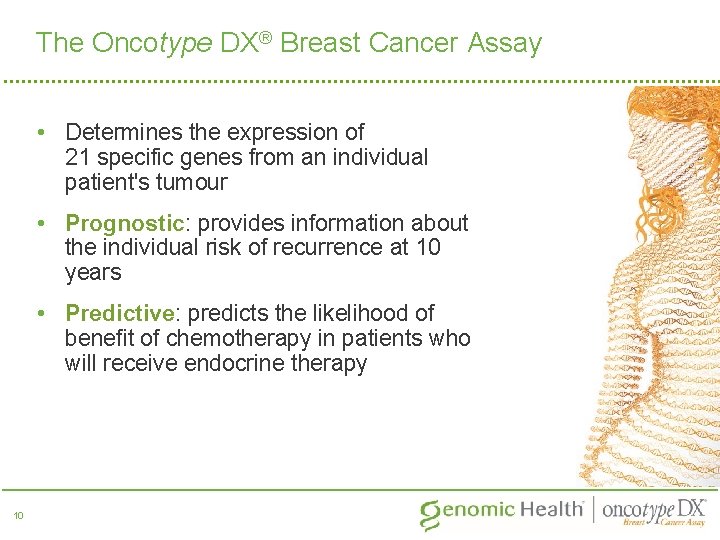
The Oncotype DX® Breast Cancer Assay • Determines the expression of 21 specific genes from an individual patient's tumour • Prognostic: provides information about the individual risk of recurrence at 10 years • Predictive: predicts the likelihood of benefit of chemotherapy in patients who will receive endocrine therapy 10
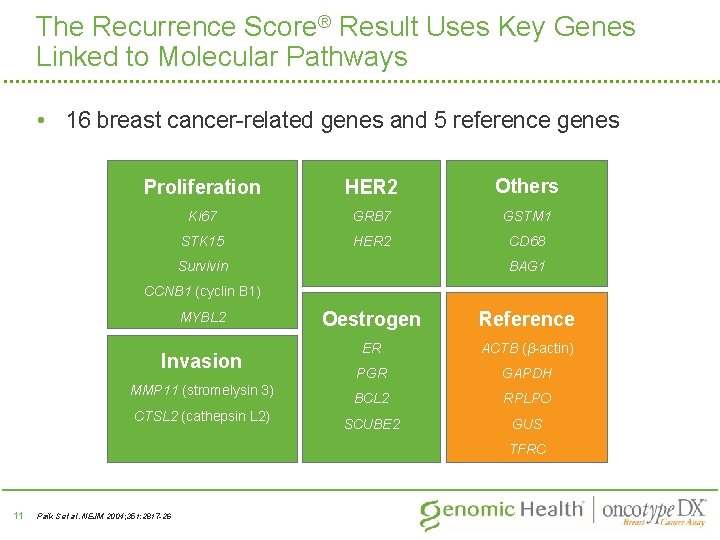
The Recurrence Score® Result Uses Key Genes Linked to Molecular Pathways • 16 breast cancer-related genes and 5 reference genes Proliferation HER 2 Others Ki 67 GRB 7 GSTM 1 STK 15 HER 2 CD 68 BAG 1 Survivin CCNB 1 (cyclin B 1) MYBL 2 Invasion MMP 11 (stromelysin 3) CTSL 2 (cathepsin L 2) Oestrogen Reference ER ACTB (β-actin) PGR GAPDH BCL 2 RPLPO SCUBE 2 GUS TFRC 11 Paik S et al. NEJM 2004; 351: 2817 -26
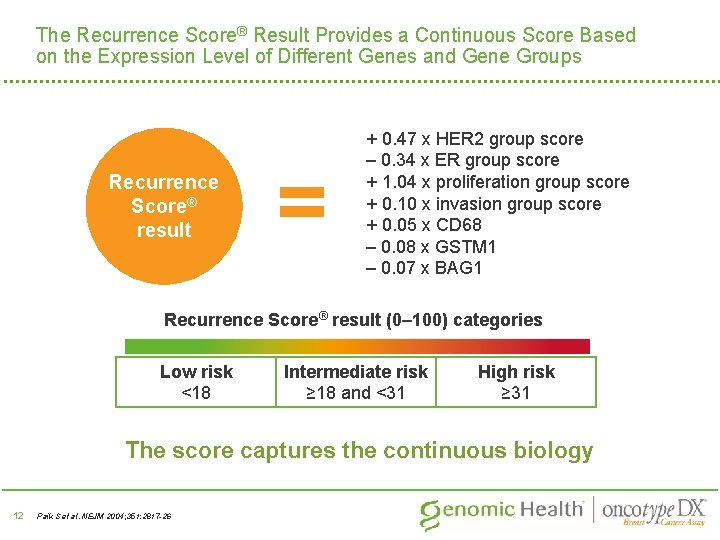
The Recurrence Score® Result Provides a Continuous Score Based on the Expression Level of Different Genes and Gene Groups Recurrence Score® result = + 0. 47 x HER 2 group score – 0. 34 x ER group score + 1. 04 x proliferation group score + 0. 10 x invasion group score + 0. 05 x CD 68 – 0. 08 x GSTM 1 – 0. 07 x BAG 1 Recurrence Score® result (0– 100) categories Low risk <18 Intermediate risk ≥ 18 and <31 High risk ≥ 31 The score captures the continuous biology 12 Paik S et al. NEJM 2004; 351: 2817 -26
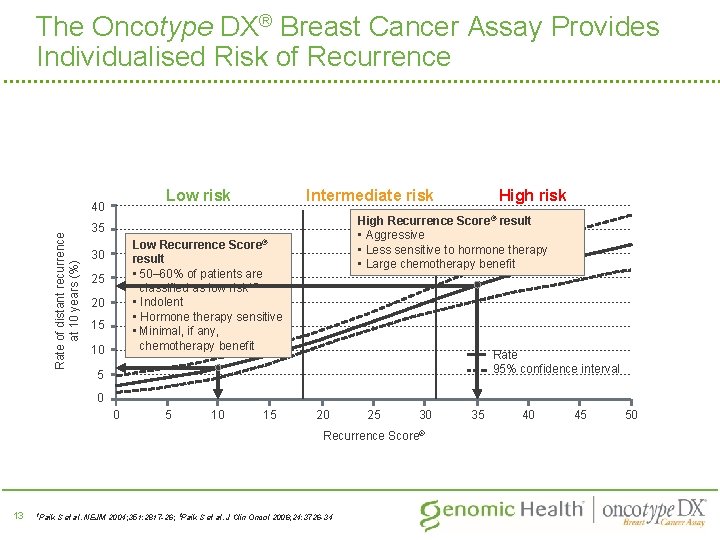
The Oncotype DX® Breast Cancer Assay Provides Individualised Risk of Recurrence Rate of distant recurrence at 10 years (%) Intermediate risk Low risk 40 High Recurrence Score® result • Aggressive • Less sensitive to hormone therapy • Large chemotherapy benefit 35 Low Recurrence Score® result • 50– 60% of patients are classified as low risk 1, 2 • Indolent • Hormone therapy sensitive • Minimal, if any, chemotherapy benefit 30 25 20 15 10 High risk Rate 95% confidence interval 5 0 0 5 10 15 20 25 30 Recurrence Score® 13 1 Paik S et al. NEJM 2004; 351: 2817 -26; 2 Paik S et al. J Clin Oncol 2006; 24: 3726 -34 35 40 45 50
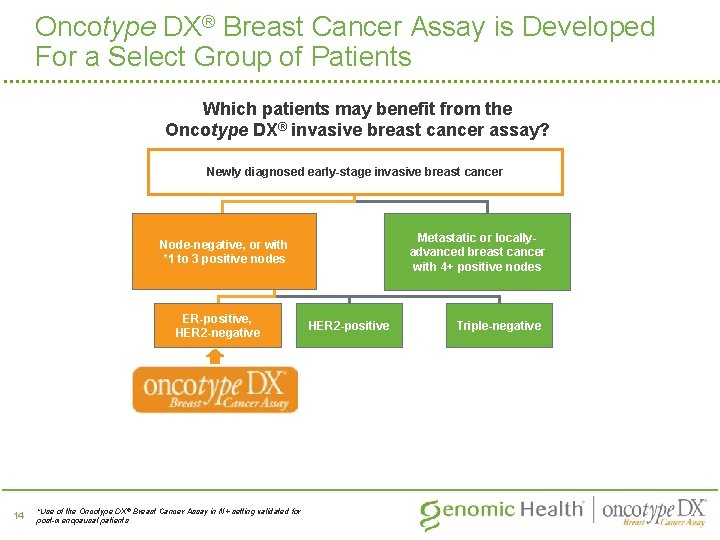
Oncotype DX® Breast Cancer Assay is Developed For a Select Group of Patients Which patients may benefit from the Oncotype DX® invasive breast cancer assay? Newly diagnosed early-stage invasive breast cancer Metastatic or locallyadvanced breast cancer with 4+ positive nodes Node-negative, or with *1 to 3 positive nodes ER-positive, HER 2 -negative 14 *Use of the Oncotype DX ® Breast Cancer Assay in N+ setting validated for post-menopausal patients HER 2 -positive Triple-negative
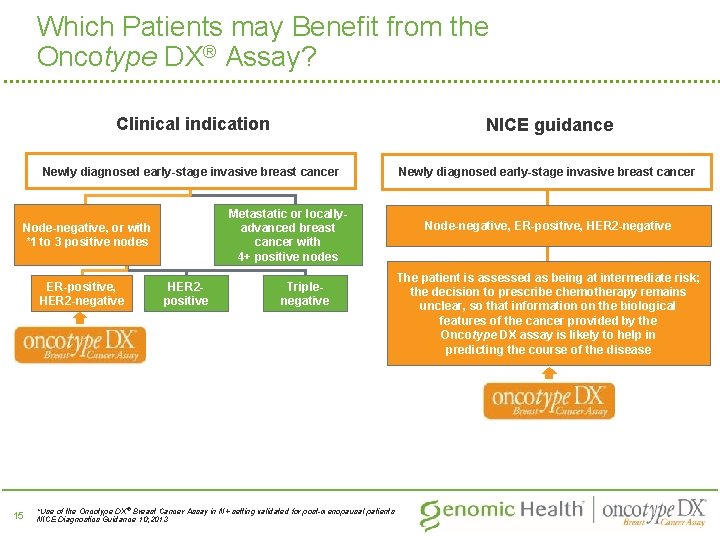
Which Patients may Benefit from the Oncotype DX® Assay? Clinical indication NICE guidance Newly diagnosed early-stage invasive breast cancer Metastatic or locallyadvanced breast cancer with 4+ positive nodes Node-negative, or with *1 to 3 positive nodes ER-positive, HER 2 -negative 15 HER 2 positive Triplenegative *Use of the Oncotype DX ® Breast Cancer Assay in N+ setting validated for post-menopausal patients NICE Diagnostics Guidance 10; 2013 Node-negative, ER-positive, HER 2 -negative The patient is assessed as being at intermediate risk; the decision to prescribe chemotherapy remains unclear, so that information on the biological features of the cancer provided by the Oncotype DX assay is likely to help in predicting the course of the disease
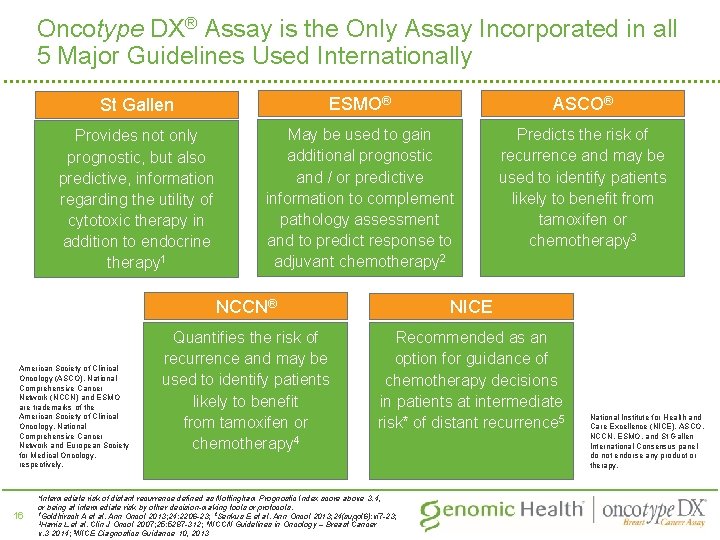
Oncotype DX® Assay is the Only Assay Incorporated in all 5 Major Guidelines Used Internationally St Gallen ESMO® ASCO® Provides not only prognostic, but also predictive, information regarding the utility of cytotoxic therapy in addition to endocrine therapy 1 May be used to gain additional prognostic and / or predictive information to complement pathology assessment and to predict response to adjuvant chemotherapy 2 Predicts the risk of recurrence and may be used to identify patients likely to benefit from tamoxifen or chemotherapy 3 American Society of Clinical Oncology (ASCO), National Comprehensive Cancer Network (NCCN) and ESMO are trademarks of the American Society of Clinical Oncology, National Comprehensive Cancer Network and European Society for Medical Oncology, respectively. 16 NCCN® NICE Quantifies the risk of recurrence and may be used to identify patients likely to benefit from tamoxifen or chemotherapy 4 Recommended as an option for guidance of chemotherapy decisions in patients at intermediate risk* of distant recurrence 5 *Intermediate risk of distant recurrence defined as Nottingham Prognostic Index score above 3. 4, or being at intermediate risk by other decision-making tools or protocols. 1 Goldhirsch A et al. Ann Oncol 2013; 24: 2206 -23; 2 Senkus E et al. Ann Oncol 2013; 24(suppl 6): vi 7 -23; 3 Harris L et al. Clin J Oncol 2007; 25: 5287 -312; 4 NCCN Guidelines in Oncology – Breast Cancer v. 3 2014; 5 NICE Diagnostics Guidance 10, 2013 National Institute for Health and Care Excellence (NICE), ASCO, NCCN, ESMO, and St Gallen International Consensus panel do not endorse any product or therapy.
The Oncotype DX® Breast Cancer Assay is Extensively Studied With Consistent Results Prognostication in endocrine-treated node-negative: • • NSABP B-141 (n=668) – tamoxifen Kaiser Permanente 2 (n=790) – tamoxifen JBCRG study 3 (n=200) – tamoxifen trans. ATAC 4 (n=872) – tamoxifen and anastrozole Prognostication in endocrine-treated node-positive: • • trans. ATAC 4 (n=306) – tamoxifen and anastrozole SWOG-88145 (n=148) – tamoxifen Prediction of chemotherapy benefit: • • NSABP B-206 (n=651) – CMF / MF in N 0 patients SWOG-88145 (n=219) – CAF in node positive patients Supportive studies in taxane versus anthracycline-treated patients: • • • 1 Paik 17 ECOG E 21977 (n=465) – N 0– 3 patients NSABP B-288 (n=1065) – node positive patients PACS 019 (n=530) – node positive patients S et al. NEJM 2004; 351: 2817 -26; 2 Habel LA et al. Breast Cancer Res 2006; 8: R 25; 3 Toi M et al. Cancer 2010; 116: 3112 -8; 4 Dowsett M et al. J Clin Oncol 2010; 28: 1829 -34; 5 Albain KS et al. Lancet Oncol 2010; 11: 55 -65; 6 Paik S et al. J Clin Oncol 2006; 24: 3726 -34 ; 7 Goldstein LJ et al. J Clin Oncol 2008; 26: 4063 -71; 8 Mamounas EP et al. ASCO 2012; abstr 1; 9 Penault-Llorca F et al. ASCO 2014; poster 11052
The Oncotype DX® Breast Cancer Assay is Extensively Studied With Consistent Results Prediction of late recurrences: • NSABP B-28 (n=1065) and B-14 (n=668)13 Neoadjuvant chemotherapy studies: • • Gianni et al 1 (n=89) Chang et al 2 (n=72) Yardley et al 3 (n=168) Pivot et al 4 (n=81) Neoadjuvant endocrine studies: • • Akashi-Tanaka et al 5 (n=43) Masuda et al 6 (n=77) ER / PR / HER 2 concordance RT-PCR / IHC / FISH: • • ER / PR 7 -8 HER 29 -11 Prediction of tamoxifen benefit: • 1 Gianni 18 Kim et al 12 (n=645) L et al. J Clin Oncol 2005; 23: 7265 -77; 2 Chang JC et al. Breast Cancer Res Treat 2008; 108: 233 -40; 3 Yardley DA et al. SABCS 2011; abstr P 5 -13 -09; 4 Pivot Xet al EBCC 2014; poster 47; 5 Akashi-Tanaka S et al. Breast 2009; 18: 171 -4; 6 Masuda N et al. ASCO 2011; poster 558; 7 Baehner FL et al. SABCS 2007; poster P 1 -07 -11; 8 Badve SS et al. J Clin Oncol 2008; 26: 2473 -81; 9 Baehner FL et al. J Clin Oncol 2010; 28: 4300 -06; 10 Sparano JL et al. ASCO 2008; abstr 13; 11 Perez EA et al. ASCO 2013; abstr 520 ; 12 Kim C et al. J Clin Oncol 2011; 29: 4160 -7; 13 Wolmark N et al. ASCO 2014; poster 11024.
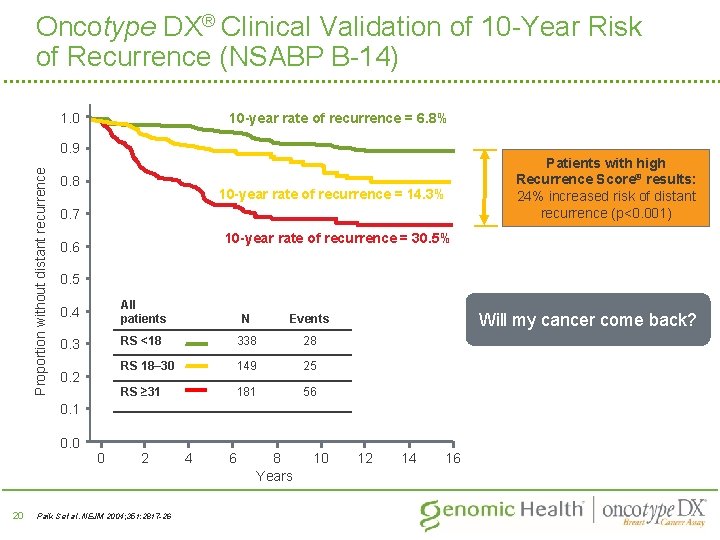
Oncotype DX® Clinical Validation of 10 -Year Risk of Recurrence (NSABP B-14) 10 -year rate of recurrence = 6. 8% 1. 0 Proportion without distant recurrence 0. 9 0. 8 10 -year rate of recurrence = 14. 3% 0. 7 10 -year rate of recurrence = 30. 5% 0. 6 0. 5 0. 4 All patients N Events 0. 3 RS <18 338 28 RS 18– 30 149 25 RS ≥ 31 181 56 0. 2 Will my cancer come back? 0. 1 0. 0 20 Patients with high Recurrence Score® results: 24% increased risk of distant recurrence (p<0. 001) 0 2 Paik S et al. NEJM 2004; 351: 2817 -26 4 6 8 Years 10 12 14 16
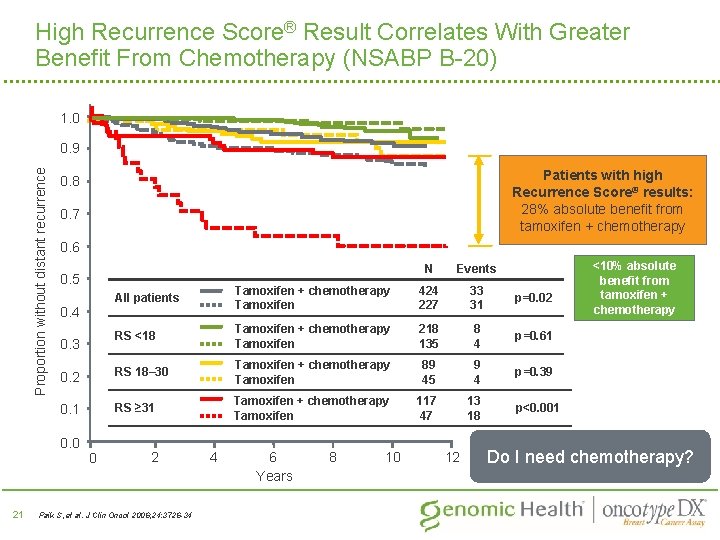
High Recurrence Score® Result Correlates With Greater Benefit From Chemotherapy (NSABP B-20) 1. 0 Proportion without distant recurrence 0. 9 0. 7 0. 6 0. 5 N Events All patients Tamoxifen + chemotherapy Tamoxifen 424 227 33 31 p=0. 02 RS <18 Tamoxifen + chemotherapy Tamoxifen 218 135 8 4 p=0. 61 0. 2 RS 18– 30 Tamoxifen + chemotherapy Tamoxifen 89 45 9 4 p=0. 39 0. 1 RS ≥ 31 Tamoxifen + chemotherapy Tamoxifen 117 47 13 18 0. 4 0. 3 0. 0 21 Patients with high Recurrence Score® results: 28% absolute benefit from tamoxifen + chemotherapy 0. 8 0 2 Paik S, et al. J Clin Oncol 2006; 24: 3726 -34 4 6 Years 8 10 12 <10% absolute benefit from tamoxifen + chemotherapy p<0. 001 Do I need chemotherapy?
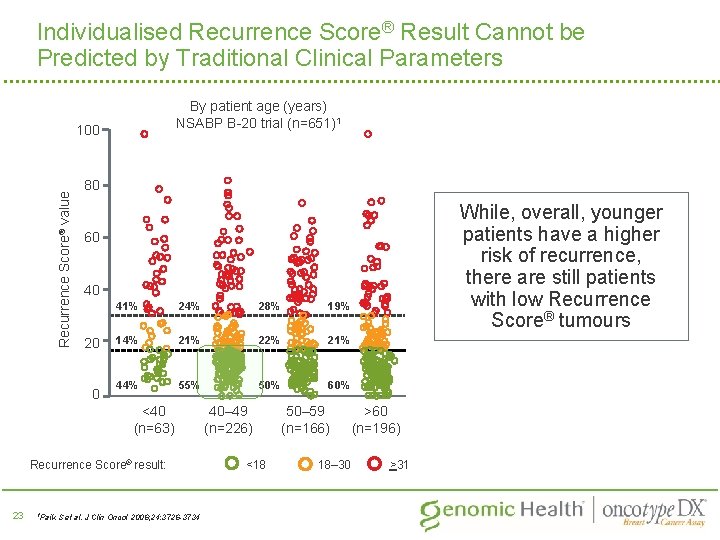
Individualised Recurrence Score® Result Cannot be Predicted by Traditional Clinical Parameters By patient age (years) NSABP B-20 trial (n=651)1 Recurrence Score® value 100 80 60 40 20 0 41% 24% 28% 19% 14% 21% 22% 21% 44% 55% 50% 60% <40 (n=63) Recurrence Score® result: 23 1 Paik While, overall, younger patients have a higher risk of recurrence, there are still patients with low Recurrence Score® tumours S et al. J Clin Oncol 2006; 24: 3726 -3734 40– 49 (n=226) <18 50– 59 (n=166) 18– 30 >60 (n=196) >31
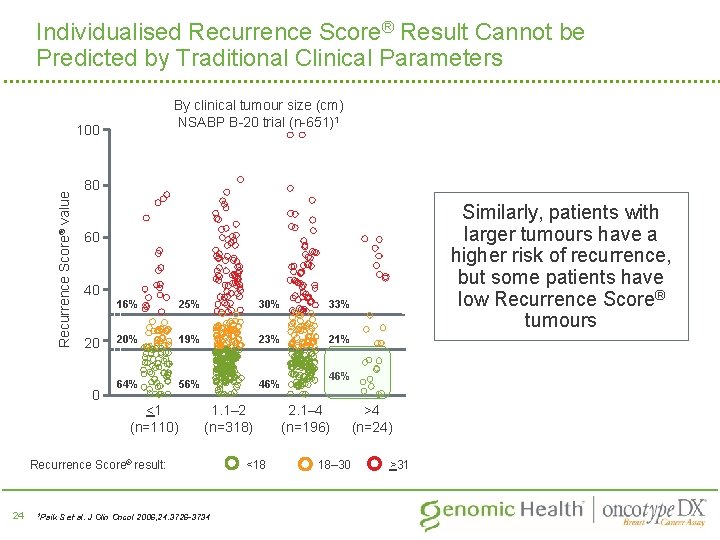
Individualised Recurrence Score® Result Cannot be Predicted by Traditional Clinical Parameters By clinical tumour size (cm) NSABP B-20 trial (n-651)1 Recurrence Score® value 100 80 60 40 20 0 16% 25% 30% 33% 20% 19% 23% 21% 64% 56% 46% <1 (n=110) 1. 1– 2 (n=318) Recurrence Score® result: 24 1 Paik Similarly, patients with larger tumours have a higher risk of recurrence, but some patients have low Recurrence Score® tumours S et al. J Clin Oncol 2006; 24: 3726 -3734 <18 46% 2. 1– 4 (n=196) 18– 30 >4 (n=24) >31
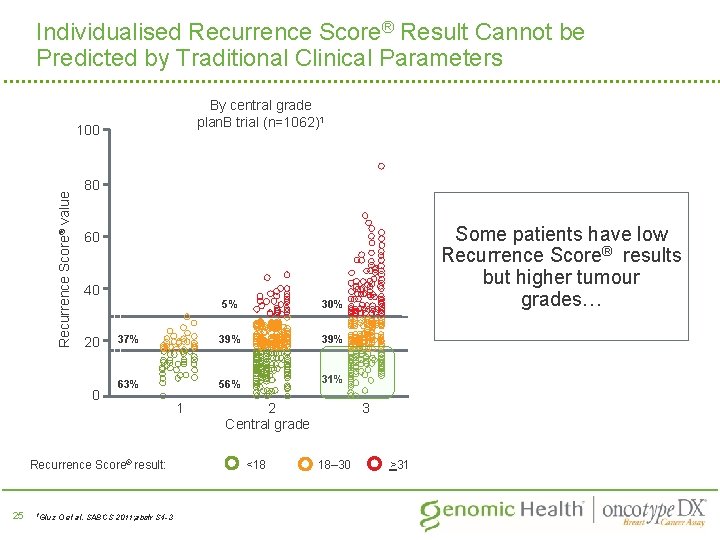
Individualised Recurrence Score® Result Cannot be Predicted by Traditional Clinical Parameters By central grade plan. B trial (n=1062)1 Recurrence Score® value 100 80 40 20 0 5% 30% 37% 39% 63% 56% 31% Recurrence Score® result: 25 1 Gluz Some patients have low Recurrence Score® results but higher tumour grades… 60 O et al. SABCS 2011; abstr S 4 -3 1 2 Central grade <18 3 18– 30 >31
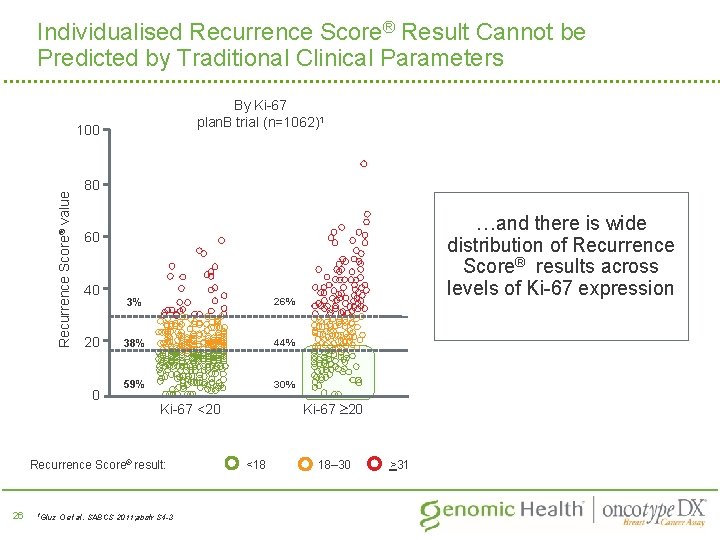
Individualised Recurrence Score® Result Cannot be Predicted by Traditional Clinical Parameters By Ki-67 plan. B trial (n=1062)1 Recurrence Score® value 100 80 40 20 0 3% 26% 38% 44% 59% 30% 1 Gluz Ki-67 20 Ki-67 <20 Recurrence Score® result: 26 …and there is wide distribution of Recurrence Score® results across levels of Ki-67 expression 60 O et al. SABCS 2011; abstr S 4 -3 <18 18– 30 >31
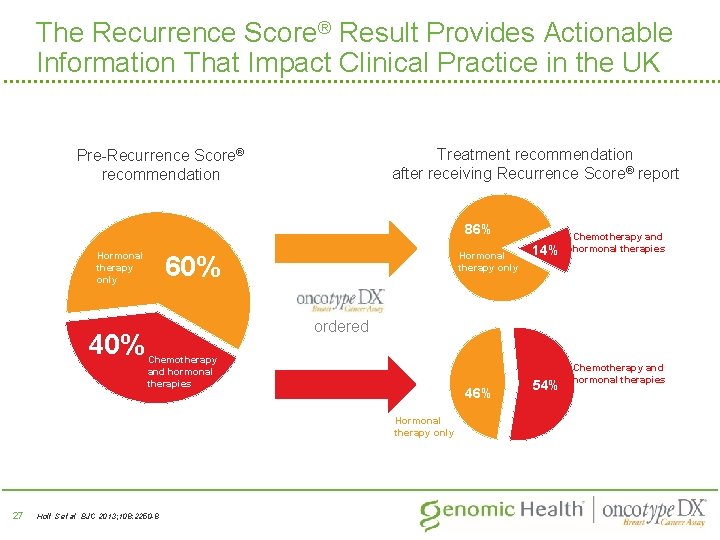
The Recurrence Score® Result Provides Actionable Information That Impact Clinical Practice in the UK Treatment recommendation after receiving Recurrence Score® report Pre-Recurrence Score® recommendation 86% Hormonal therapy only 40% 82% Hormonal therapy only 60% ordered Chemotherapy and hormonal therapies 46% Hormonal therapy only 27 14% Chemotherapy and hormonal therapies Holt S et al BJC 2013; 108: 2250 -8 54% Chemotherapy and hormonal therapies
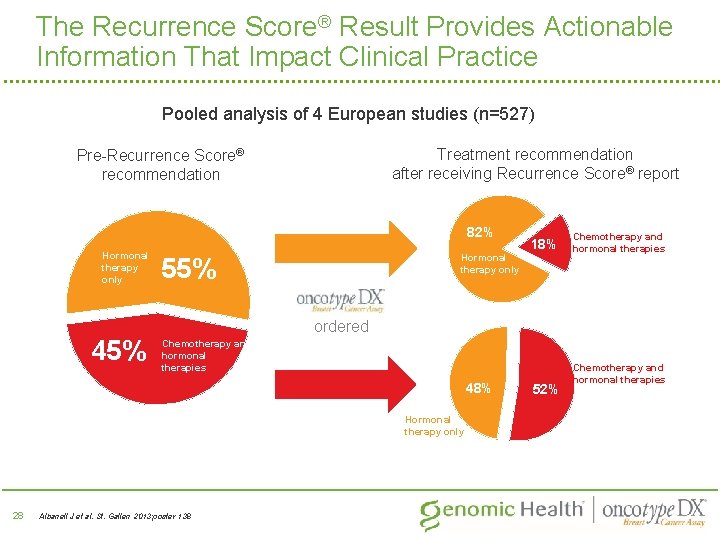
The Recurrence Score® Result Provides Actionable Information That Impact Clinical Practice Pooled analysis of 4 European studies (n=527) Treatment recommendation after receiving Recurrence Score® report Pre-Recurrence Score® recommendation 82% Hormonal therapy only 45% 82% Hormonal therapy only 55% Chemotherapy and hormonal therapies ordered Chemotherapy and hormonal therapies 48% Hormonal therapy only 28 18% Albanell J et al. St. Gallen 2013; poster 138 52% Chemotherapy and hormonal therapies
Oncotype DX® Breast Cancer Assay • Intended for newly diagnosed patients with early-stage ER+, HER 2 -, invasive breast cancer, helping to make the best possible treatment decision for every patient. • The ONLY multi-gene assay that quantifies risk of disease recurrence and likelihood of benefit from chemotherapy. • The ONLY assay consistently incorporated into the 5 internationally recognized clinical guidelines (ASCO, NCCN, St. Gallen, ESMO, and NICE ) based on strong clinical evidence, a high level of standardisation and cost effectiveness. • A proven 10 year track record with over 450, 000 patients with breast cancer tested worldwide. 30

The Oncotype DX® Assay in Clinical Practice
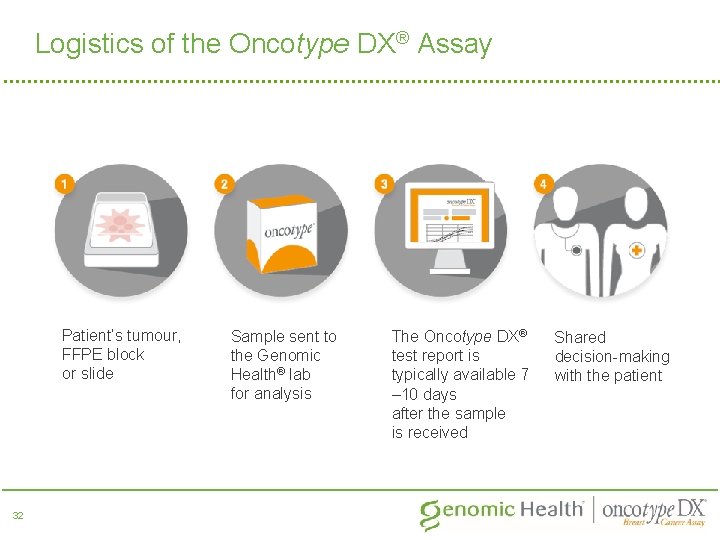
Logistics of the Oncotype DX® Assay Patient’s tumour, FFPE block or slide 32 Sample sent to the Genomic Health® lab for analysis The Oncotype DX® test report is typically available 7 – 10 days after the sample is received Shared decision-making with the patient

Central State-of-the-Art Laboratory Central state-of-the-art laboratory with highly standardised quality control processes Consistent accuracy and high reproducibility 1 of test results over time 2 33 1 Cronin M et al. Clin Chem 2007; 53: 1084 -91; 2 Baehner FL et al. San Antonio Breast Cancer Symposium 2011 P 1 -07 -11
The Oncotype DX® Patient Report I • Example of patient report for ER+, HER 2 -, N 0, invasive breast cancer patient • The patient report includes prognostic information: average rate of distant recurrence at 10 years 34
The Oncotype DX® Patient Report II • Example of patient report for ER+, HER 2 -, N 0, invasive breast cancer patient • The patient report includes predictive information: the likelihood of benefit from additional chemotherapy 35
The Oncotype DX® Patient Report III • Example of patient report for ER+, HER 2 -, N 0, invasive breast cancer patient • The patient report includes a continuous, quantitative, single gene report of ER, PR and HER 2 36

Patient Cases
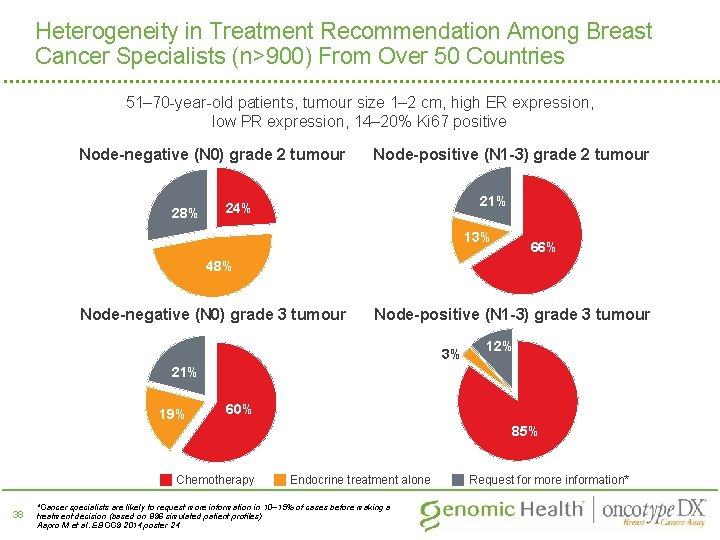
Heterogeneity in Treatment Recommendation Among Breast Cancer Specialists (n>900) From Over 50 Countries 51– 70 -year-old patients, tumour size 1– 2 cm, high ER expression, low PR expression, 14– 20% Ki 67 positive Node-negative (N 0) grade 2 tumour 28% Node-positive (N 1 -3) grade 2 tumour 21% 24% 13% 66% 48% Node-negative (N 0) grade 3 tumour Node-positive (N 1 -3) grade 3 tumour 3% 12% 21% 19% 60% 85% Chemotherapy 38 Endocrine treatment alone *Cancer specialists are likely to request more information in 10– 15% of cases before making a treatment decision (based on 896 simulated patient profiles) Aapro M et al. EBCC 9 2014; poster 24 Request for more information*
39
Summary of the Oncotype DX® Assay for ER+, HER 2 -, Early Stage Invasive Breast Cancer Patients • The only multigene assay with demonstrated information regarding: • • Prognostication (average rate of distant recurrence at 10 -year risk)1 Prediction of chemotherapy benefit 2 • Oncotype DX® Breast Cancer Assay can reliably classify ~50% of patients at ‘low risk’ who are unlikely to benefit from adjuvant chemotherapy 1, 2 • Oncotype DX® Breast Cancer Assay has been extensively assessed in 14 studies with over 6000 invasive breast cancer patients with consistent results (Level I, Category B evidence)3, 4 • The only multigene assay incorporated into 5 major clinical guidelines (St Gallen, ESMO®, ASCO®, NCCN® and NICE)5 -9 1 Paik 40 S et al. NEJM 2004; 351: 2817 -26; 2 Paik S et al J Clin Oncol 2006; 24: 3726 -34; 3 Hornberger J et al. JNCI 2012; 104: 1068 -79; 4 Burke E et al. EBCC 9 2014; poster 525; 5 Goldhirsch A et al. Ann Oncol 2013; 24: 2206 -23; 6 Senkus E et al. Ann Oncol 2013; 24(suppl 6): vi 7 -23; 7 Harris L et al. Clin J Oncol 2007; 25: 5287 -312; 8 NCCN Guidelines in Oncology – Breast Cancer v. 3 2014; 9 NICE Diagnostics Guidance 10, 2013

Thanks for your attention
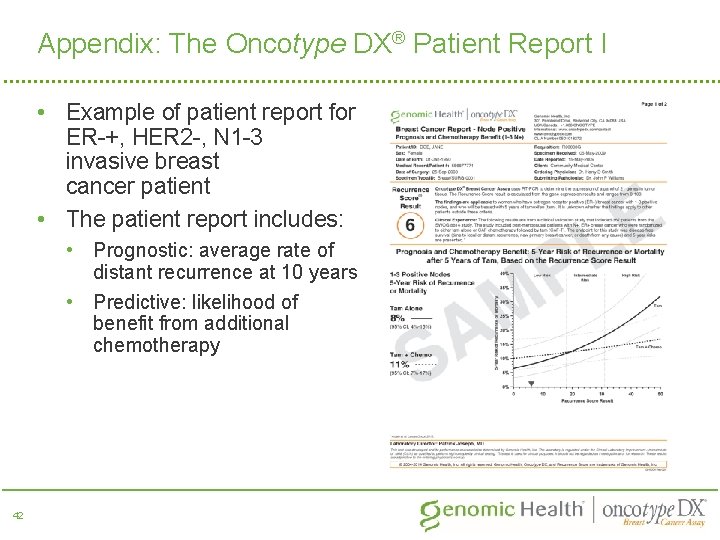
Appendix: The Oncotype DX® Patient Report I • Example of patient report for ER-+, HER 2 -, N 1 -3 invasive breast cancer patient • The patient report includes: • Prognostic: average rate of distant recurrence at 10 years • Predictive: likelihood of benefit from additional chemotherapy 42
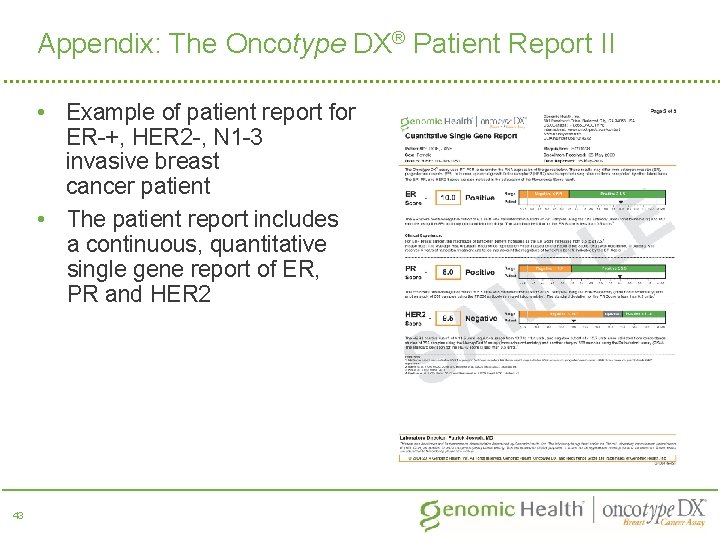
Appendix: The Oncotype DX® Patient Report II • Example of patient report for ER-+, HER 2 -, N 1 -3 invasive breast cancer patient • The patient report includes a continuous, quantitative single gene report of ER, PR and HER 2 43
Appendix: Endocrine Therapy is Effective for ER+, HER 2 - Patients, but is not Sufficient for all Patients • Substantial benefit of adjuvant tamoxifen therapy in ER-positive patients (16% gain on the 10 -year probability of distant recurrence compared with no treatment)1 • However, there are still 19% of ER+, HER 2 -, lymph nodenegative patients treated with surgery and endocrine therapy who will recur 1 44 1 EBCTCG. Lancet 2011; 378: 771 -84
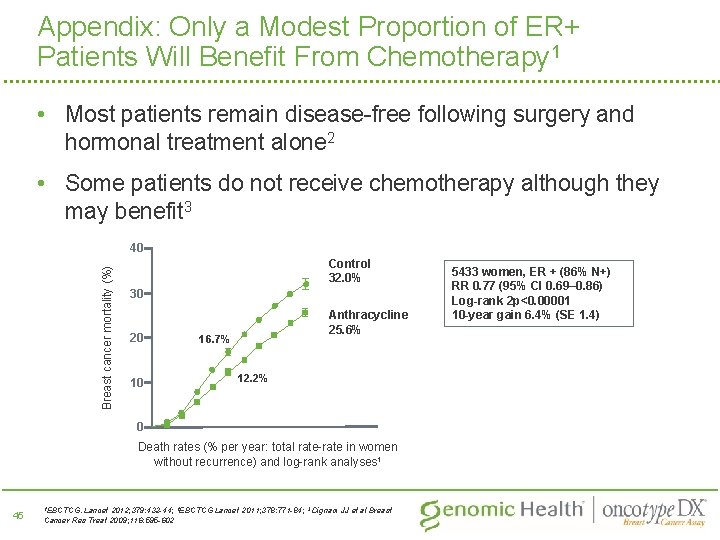
Appendix: Only a Modest Proportion of ER+ Patients Will Benefit From Chemotherapy 1 • Most patients remain disease-free following surgery and hormonal treatment alone 2 • Some patients do not receive chemotherapy although they may benefit 3 Breast cancer mortality (%) 40 Control 32. 0% 30 20 10 Anthracycline 25. 6% 16. 7% 12. 2% 0 Death rates (% per year: total rate-rate in women without recurrence) and log-rank analyses 1 45 1 EBCTCG. Lancet 2012; 379: 432 -44; 2 EBCTCG Lancet 2011; 378: 771 -84; 3 Dignam JJ et al Breast Cancer Res Treat 2009; 116: 595 -602 5433 women, ER + (86% N+) RR 0. 77 (95% CI 0. 69– 0. 86) Log-rank 2 p<0. 00001 10 -year gain 6. 4% (SE 1. 4)
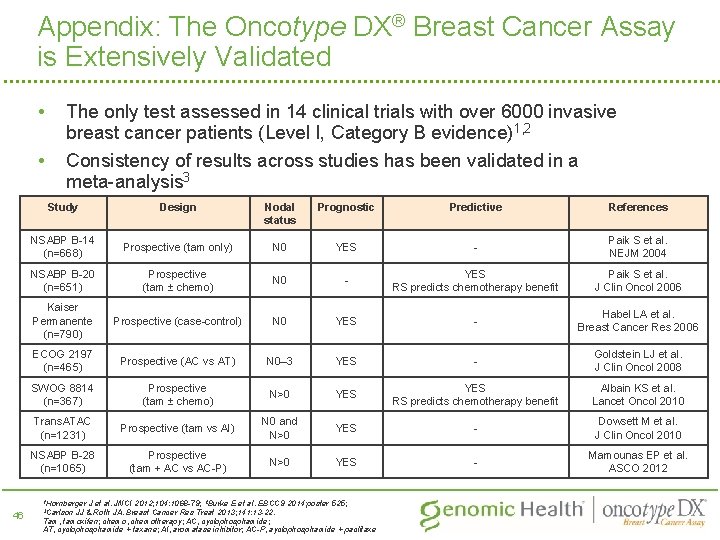
Appendix: The Oncotype DX® Breast Cancer Assay is Extensively Validated • The only test assessed in 14 clinical trials with over 6000 invasive breast cancer patients (Level I, Category B evidence)1, 2 • Consistency of results across studies has been validated in a meta-analysis 3 Study Design Nodal status Prognostic Predictive References NSABP B-14 (n=668) Prospective (tam only) N 0 YES - Paik S et al. NEJM 2004 NSABP B-20 (n=651) Prospective (tam ± chemo) N 0 - YES RS predicts chemotherapy benefit Paik S et al. J Clin Oncol 2006 Kaiser Permanente (n=790) Prospective (case-control) N 0 YES - Habel LA et al. Breast Cancer Res 2006 ECOG 2197 (n=465) Prospective (AC vs AT) N 0– 3 YES - Goldstein LJ et al. J Clin Oncol 2008 SWOG 8814 (n=367) Prospective (tam ± chemo) N>0 YES RS predicts chemotherapy benefit Albain KS et al. Lancet Oncol 2010 Trans. ATAC (n=1231) Prospective (tam vs AI) N 0 and N>0 YES - Dowsett M et al. J Clin Oncol 2010 NSABP B-28 (n=1065) Prospective (tam + AC vs AC-P) N>0 YES - Mamounas EP et al. ASCO 2012 1 Hornberger 46 J et al. JNCI 2012; 104: 1068 -79; 2 Burke E et al. EBCC 9 2014; poster 525; JJ & Roth JA. Breast Cancer Res Treat 2013; 141: 13 -22. Tam, tamoxifen; chemo, chemotherapy; AC, cyclophosphamide; AT, cyclophosphamide + taxane; AI, aromatase inhibitor; AC-P, ayclophosphamide + paclitaxe 3 Carlson
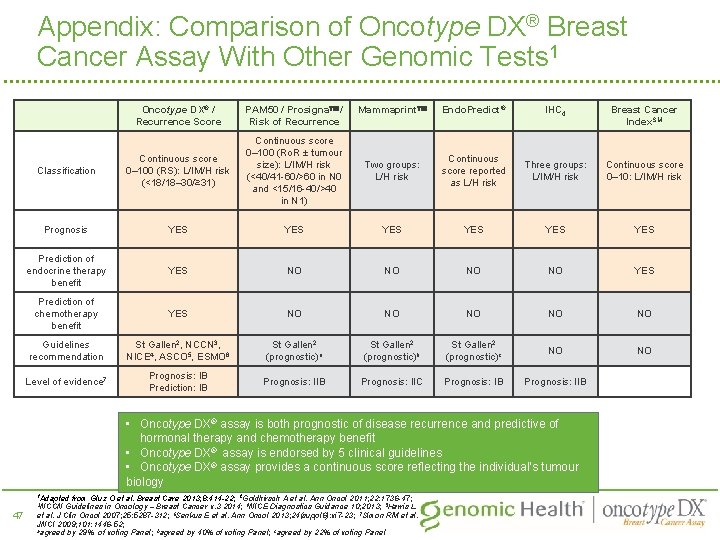
Appendix: Comparison of Oncotype DX® Breast Cancer Assay With Other Genomic Tests 1 Oncotype DX® / Recurrence Score PAM 50 / Prosigna / Risk of Recurrence Mammaprint Endo. Predict® IHC 4 Breast Cancer Index. SM Continuous score 0– 100 (RS): L/IM/H risk (<18/18– 30/≥ 31) Continuous score 0– 100 (Ro. R ± tumour size): L/IM/H risk (<40/41 -60/>60 in N 0 and <15/16 -40/>40 in N 1) Classification Two groups: L/H risk Continuous score reported as L/H risk Three groups: L/IM/H risk Continuous score 0– 10: L/IM/H risk Prognosis YES YES YES Prediction of endocrine therapy benefit YES NO NO YES Prediction of chemotherapy benefit YES NO NO NO Guidelines recommendation St Gallen 2, NCCN 3, NICE 4, ASCO 5, ESMO 6 St Gallen 2 (prognostic)a St Gallen 2 (prognostic)b St Gallen 2 (prognostic)c NO NO Level of evidence 7 Prognosis: IB Prediction: IB Prognosis: IIC Prognosis: IB Prognosis: IIB • Oncotype DX® assay is both prognostic of disease recurrence and predictive of hormonal therapy and chemotherapy benefit • Oncotype DX® assay is endorsed by 5 clinical guidelines • Oncotype DX® assay provides a continuous score reflecting the individual’s tumour biology 1 Adapted 47 from Gluz O et al. Breast Care 2013; 8: 414 -22; 2 Goldhirsch A et al. Ann Oncol 2011; 22: 1736 -47; Guidelines in Oncology – Breast Cancer v. 3 2014; 4 NICE Diagnostics Guidance 10; 2013; 5 Harris L et al. J Clin Oncol 2007; 25: 5287 -312; 6 Senkus E et al. Ann Oncol 2013; 24(suppl 6): vi 7 -23; 7 Simon RM et al. JNCI 2009; 101: 1446 -52; aagreed by 29% of voting Panel; bagreed by 40% of voting Panel; cagreed by 22% of voting Panel 3 NCCN
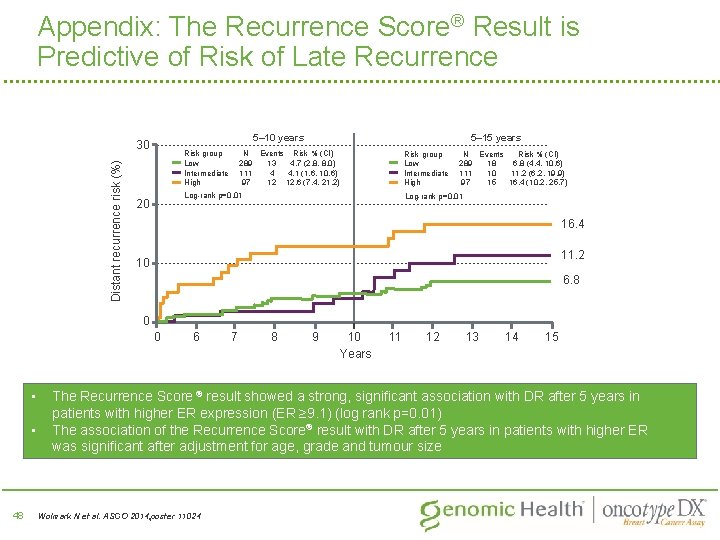
Appendix: The Recurrence Score® Result is Predictive of Risk of Late Recurrence 5– 10 years Distant recurrence risk (%) 30 Risk group Low Intermediate High 5– 15 years N Events Risk % (CI) 289 13 4. 7 (2. 8, 8. 0) 111 4 4. 1 (1. 6, 10. 6) 97 12 12. 6 (7. 4, 21. 2) Risk group Low Intermediate High Log-rank p=0. 01 20 N Events Risk % (CI) 289 18 6. 8 (4. 4, 10. 6) 111 10 11. 2 (6. 2, 19. 9) 97 15 16. 4 (10. 2, 25. 7) Log-rank p=0. 01 16. 4 11. 2 10 6. 8 0 0 • • 48 6 7 8 9 10 Years 11 12 13 14 15 The Recurrence Score ® result showed a strong, significant association with DR after 5 years in patients with higher ER expression (ER ≥ 9. 1) (log rank p=0. 01) The association of the Recurrence Score® result with DR after 5 years in patients with higher ER was significant after adjustment for age, grade and tumour size Wolmark N et al. ASCO 2014; poster 11024
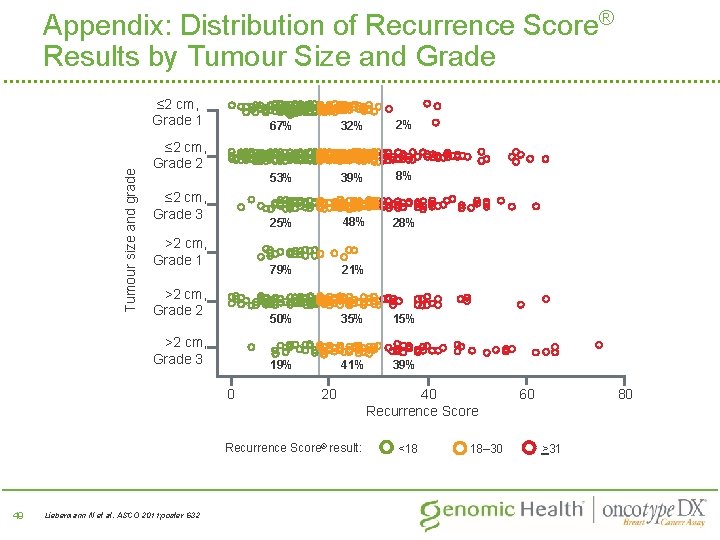
Appendix: Distribution of Recurrence Score® Results by Tumour Size and Grade Tumour size and grade ≤ 2 cm, Grade 1 ≤ 2 cm, Grade 2 ≤ 2 cm, Grade 3 67% 32% 2% 53% 39% 8% 25% 48% 21% 79% (n=29) >2 cm, Grade 2 50% 35% 15% (n=183) 19% 0 41% 20 Recurrence Score® result: Liebermann N et al. ASCO 2011; poster 632 (n=775) (n=194) >2 cm, Grade 1 >2 cm, Grade 3 49 (n=247) 39% 40 Recurrence Score <18 18– 30 60 80 >31
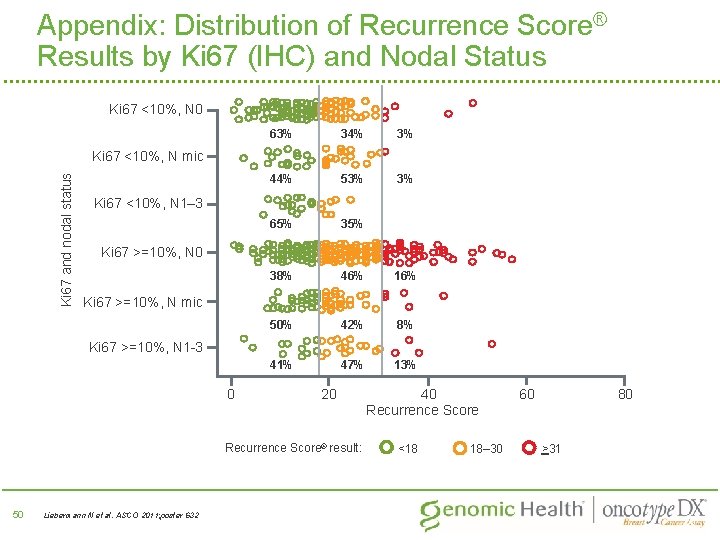
Appendix: Distribution of Recurrence Score® Results by Ki 67 (IHC) and Nodal Status Ki 67 <10%, N 0 63% 34% 3% 44% 53% 3% 65% 38% 46% 16% 50% 42% 8% 41% 47% 13% Ki 67 and nodal status Ki 67 <10%, N mic Ki 67 <10%, N 1– 3 Ki 67 >=10%, N 0 Ki 67 >=10%, N mic Ki 67 >=10%, N 1 -3 0 20 Recurrence Score® result: 50 Liebermann N et al. ASCO 2011; poster 632 Liebermann et al. ESMO 2011 40 Recurrence Score <18 18– 30 60 80 >31 50
Appendix: The Oncotype DX® Breast Cancer Assay Positively Impacts Quality of Life and is Cost Saving • Long-term clinical and costs evaluation estimates that use of the Oncotype DX® results in an increased mean life expectancy of 0. 16 years and an increased quality-adjusted life expectancy of 0. 14 years per patient, compared with current clinical practice 1 • Routine use of the Oncotype DX® assay is cost-effective and cost-saving versus conventional approaches 2 • Benefits driven by optimising chemotherapy treatment and reducing supportive care 3, 4 • Initial upfront costs offset by reduced hospitalisation due to chemotherapy toxicity and costs of treatment following distant recurrence 4 51 1 Holt SDH et al SABCS 2011; poster PD 06 -02; 2 Rouzier R et al Breast cancer Res Treat 2013; 139: 621 -37; 3 Lamond NWC et al Expert Rev Pharmacoecon Outcomes Res 2013; 13: 243 -50; 4 Paulden M et al Value Health 2013; 16: 729 -39
- Slides: 47