The Octet Rule Noble Gases the happiest elements

The Octet Rule

Noble Gases: the happiest elements

Why are the Noble Gases happy? n Happy atoms have a full outer energy level of electrons. n They rarely combine with other elements. n Nonreactivity is why they are called noble or inert.
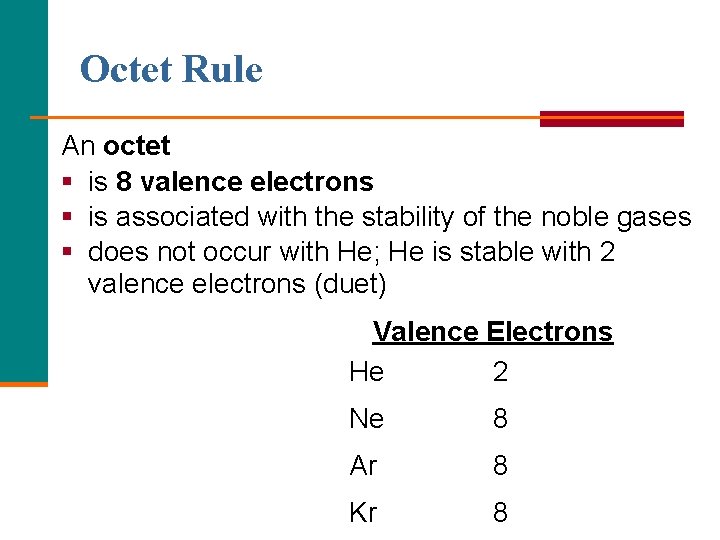
Octet Rule An octet § is 8 valence electrons § is associated with the stability of the noble gases § does not occur with He; He is stable with 2 valence electrons (duet) Valence Electrons He 2 Ne 8 Ar 8 Kr 8

Octet Rule In order to achieve an octet, elements will form ions. § An ion is an atom or group of atoms with a charge (not neutral)

Metals want to be happy. Metals form positive ions or cations § by losing their valence electrons § resemble the nearest noble gas § have fewer electrons than protons Group 1 metals Group 2 metals Group 3 metals ion 1+ ion 2+ ion 3+
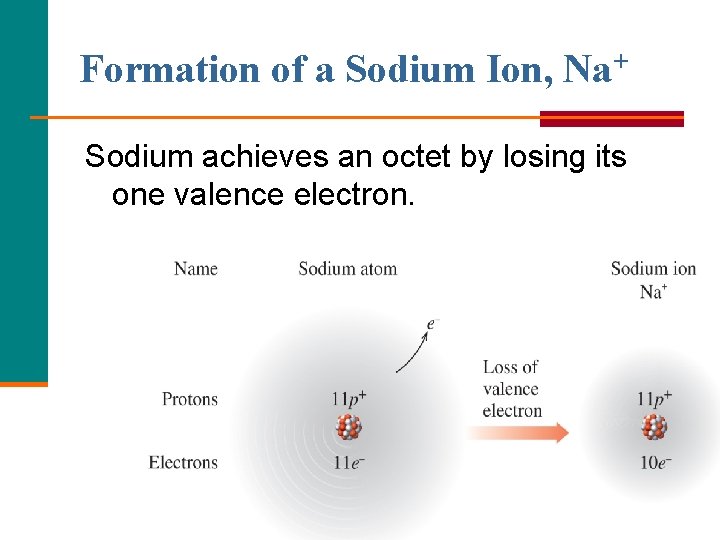
Formation of a Sodium Ion, Na+ Sodium achieves an octet by losing its one valence electron.
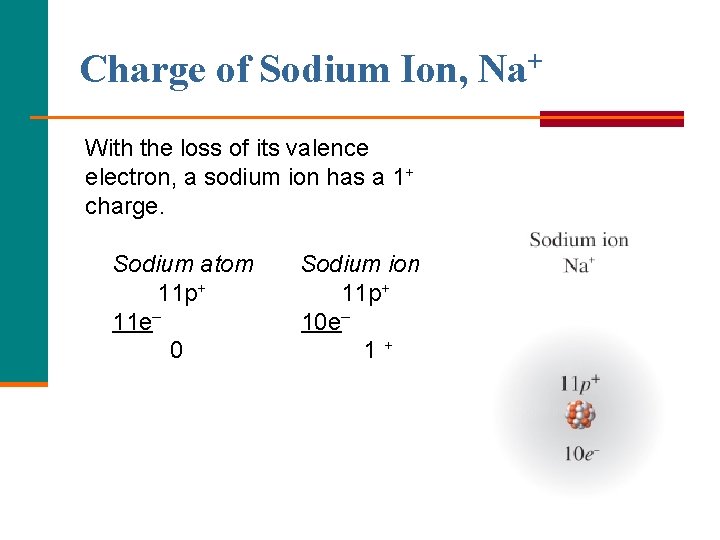
Charge of Sodium Ion, Na+ With the loss of its valence electron, a sodium ion has a 1+ charge. Sodium atom 11 p+ 11 e– 0 Sodium ion 11 p+ 10 e– 1+
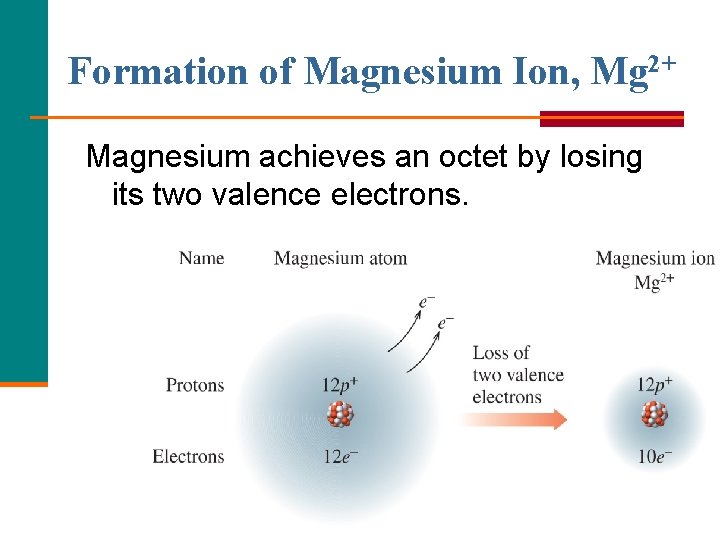
Formation of Magnesium Ion, Mg 2+ Magnesium achieves an octet by losing its two valence electrons.
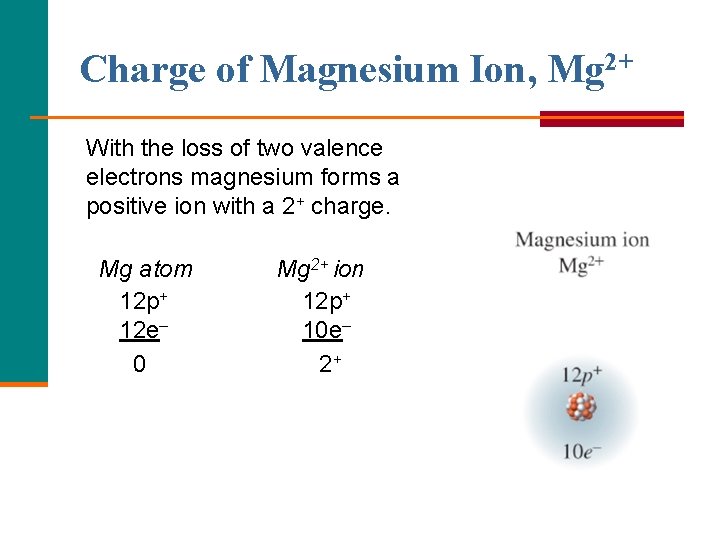
Charge of Magnesium Ion, Mg 2+ With the loss of two valence electrons magnesium forms a positive ion with a 2+ charge. Mg atom 12 p+ 12 e– 0 Mg 2+ ion 12 p+ 10 e– 2+

Nonmetals want to be happy too. Nonmetals form negative ions or anions § gain electrons § have more electrons than protons § form negatively charged ions with 3–, 2–, or 1– charges
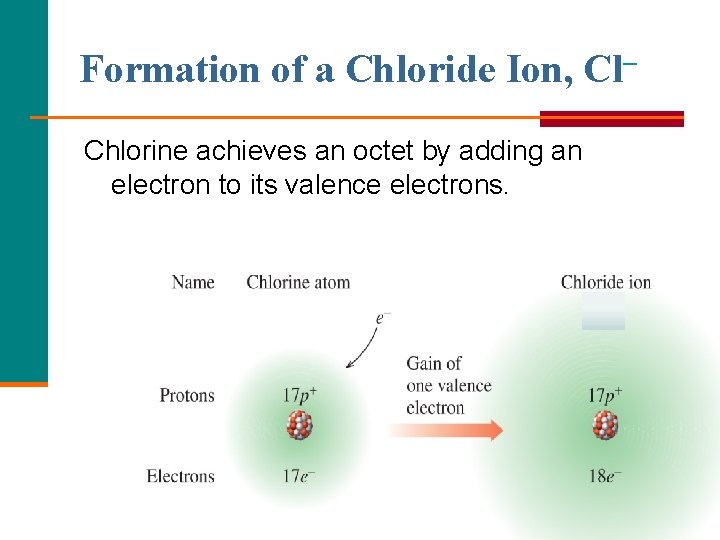
Formation of a Chloride Ion, Cl– Chlorine achieves an octet by adding an electron to its valence electrons.
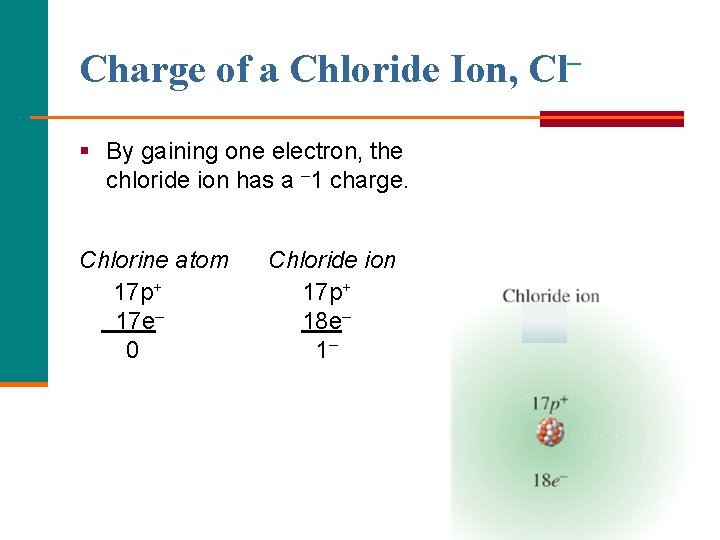
Charge of a Chloride Ion, Cl– § By gaining one electron, the chloride ion has a – 1 charge. Chlorine atom 17 p+ 17 e– 0 Chloride ion 17 p+ 18 e– 1–
- Slides: 13