THE OBJECTIVES AND DEVELOPMENT OF A JOINT NRA

THE OBJECTIVES AND DEVELOPMENT OF A JOINT NRA ASSESSMENT TOOL UPDATE ON HARMONIZATION OF WHO/PAHO NRA ASSESSMENT PROCESS FOR ASSESSMENT OF MEDICINES, VACCINES, MEDICAL DEVICES AND DIAGNOSTICS LAHOUARI BELGHARBI, WHO/EMP/HQ
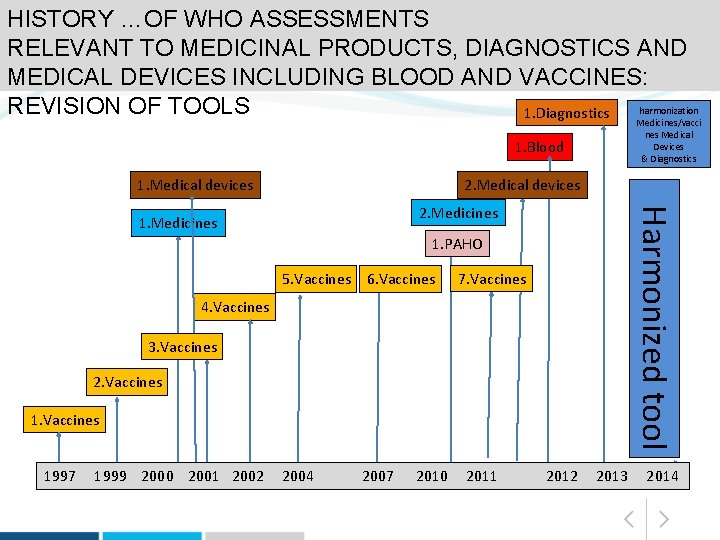
HISTORY …OF WHO ASSESSMENTS RELEVANT TO MEDICINAL PRODUCTS, DIAGNOSTICS AND MEDICAL DEVICES INCLUDING BLOOD AND VACCINES: harmonization REVISION OF TOOLS 1. Diagnostics Medicines/vacci 1. Blood 1. Medical devices 2. Medicines 1. PAHO 5. Vaccines 6. Vaccines 4. Vaccines 3. Vaccines 2. Vaccines 1. Vaccines 7. Vaccines Harmonized tool 1. Medicines Medical Devices & Diagnostics 1997 1999 2000 2001 2002 2004 2007 2010 2011 2012 2013 2014

HARMONIZED TOOL: REGULATORY FUNCTIONS 1. NATIONAL REGULATORY SYSTEM 2. REGISTRATION AND MARKETING AUTHORIZATION 3. LICENSING ACTIVITIES 4. POST-MARKETING SURVEILLANCE (+ LOT RELEASE FUNCTION) 5. OVERSIGHT OF CLINICAL TRIALS 6. INSPECTIONS AND ENFORCEMENT ACTIVITIES 7. LABORATORY ACCESS AND TESTING 8. VIGILANCE AND RISK MANAGEMENT 9. CONTROL OF PROMOTION AND ADVERTISING 10. CONTROL OF NARCOTICS, PSYCHOTROPIC SUBSTANCES AND PRECURSORS 11. PHARMACEUTICAL PERSONNEL
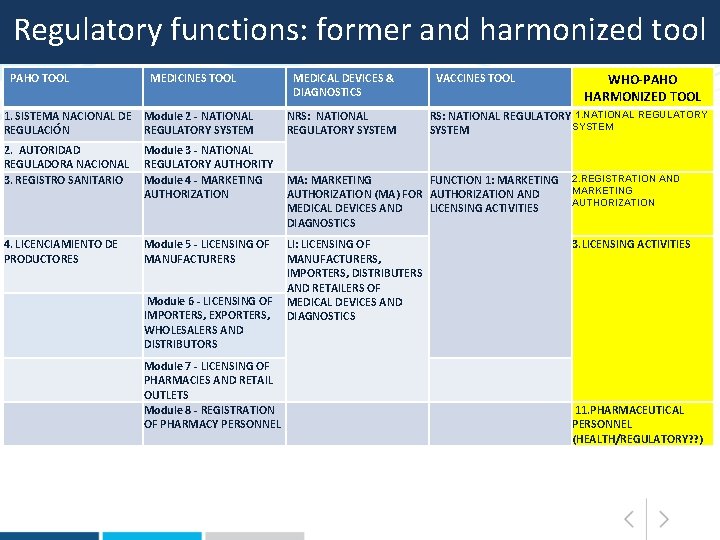
Regulatory functions: former and harmonized tool PAHO TOOL MEDICINES TOOL 1. SISTEMA NACIONAL DE Module 2 - NATIONAL REGULACIÓN REGULATORY SYSTEM 2. AUTORIDAD REGULADORA NACIONAL 3. REGISTRO SANITARIO 4. LICENCIAMIENTO DE PRODUCTORES Module 3 - NATIONAL REGULATORY AUTHORITY Module 4 - MARKETING AUTHORIZATION MEDICAL DEVICES & DIAGNOSTICS NRS: NATIONAL REGULATORY SYSTEM VACCINES TOOL WHO-PAHO HARMONIZED TOOL RS: NATIONAL REGULATORY 1. NATIONAL REGULATORY SYSTEM MA: MARKETING AUTHORIZATION (MA) FOR MEDICAL DEVICES AND DIAGNOSTICS FUNCTION 1: MARKETING 2. REGISTRATION AND MARKETING AUTHORIZATION AND AUTHORIZATION LICENSING ACTIVITIES Module 5 - LICENSING OF LI: LICENSING OF 3. LICENSING ACTIVITIES MANUFACTURERS, IMPORTERS, DISTRIBUTERS AND RETAILERS OF Module 6 - LICENSING OF MEDICAL DEVICES AND IMPORTERS, EXPORTERS, DIAGNOSTICS WHOLESALERS AND DISTRIBUTORS Module 7 - LICENSING OF PHARMACIES AND RETAIL OUTLETS Module 8 - REGISTRATION OF PHARMACY PERSONNEL 11. PHARMACEUTICAL PERSONNEL (HEALTH/REGULATORY? ? )
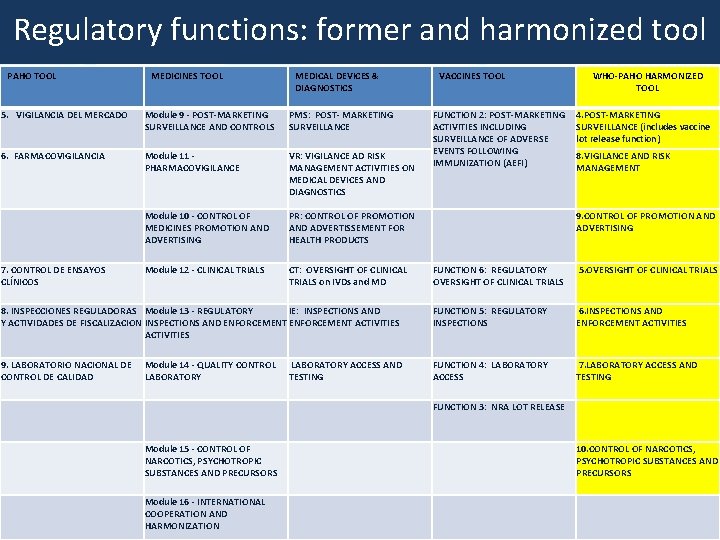
Regulatory functions: former and harmonized tool PAHO TOOL MEDICINES TOOL MEDICAL DEVICES & DIAGNOSTICS 5. VIGILANCIA DEL MERCADO Module 9 - POST-MARKETING SURVEILLANCE AND CONTROLS PMS: POST- MARKETING SURVEILLANCE 6. FARMACOVIGILANCIA Module 11 - PHARMACOVIGILANCE VR: VIGILANCE AD RISK MANAGEMENT ACTIVITIES ON MEDICAL DEVICES AND DIAGNOSTICS Module 10 - CONTROL OF MEDICINES PROMOTION AND ADVERTISING PR: CONTROL OF PROMOTION AND ADVERTISSEMENT FOR HEALTH PRODUCTS 7. CONTROL DE ENSAYOS CLÍNICOS Module 12 - CLINICAL TRIALS CT: OVERSIGHT OF CLINICAL TRIALS on IVDs and MD VACCINES TOOL FUNCTION 2: POST-MARKETING ACTIVITIES INCLUDING SURVEILLANCE OF ADVERSE EVENTS FOLLOWING IMMUNIZATION (AEFI) WHO-PAHO HARMONIZED TOOL 4. POST-MARKETING SURVEILLANCE (includes vaccine lot release function) 8. VIGILANCE AND RISK MANAGEMENT 9. CONTROL OF PROMOTION AND ADVERTISING FUNCTION 6: REGULATORY OVERSIGHT OF CLINICAL TRIALS 5. OVERSIGHT OF CLINICAL TRIALS 8. INSPECCIONES REGULADORAS Module 13 - REGULATORY IE: INSPECTIONS AND Y ACTIVIDADES DE FISCALIZACION INSPECTIONS AND ENFORCEMENT ACTIVITIES FUNCTION 5: REGULATORY INSPECTIONS 6. INSPECTIONS AND ENFORCEMENT ACTIVITIES 9. LABORATORIO NACIONAL DE CONTROL DE CALIDAD LABORATORY ACCESS AND TESTING FUNCTION 4: LABORATORY ACCESS 7. LABORATORY ACCESS AND TESTING FUNCTION 3: NRA LOT RELEASE Module 14 - QUALITY CONTROL LABORATORY Module 15 - CONTROL OF NARCOTICS, PSYCHOTROPIC SUBSTANCES AND PRECURSORS 10. CONTROL OF NARCOTICS, PSYCHOTROPIC SUBSTANCES AND PRECURSORS Module 16 - INTERNATIONAL COOPERATION AND HARMONIZATION
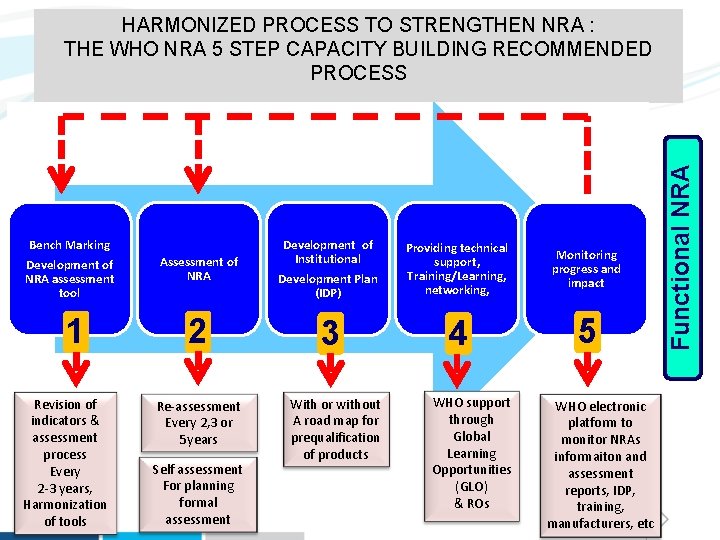
Bench Marking Development of NRA assessment tool 1 Revision of indicators & assessment process Every 2 -3 years, Harmonization of tools Assessment of NRA Development of Institutional Development Plan (IDP) Providing technical support, Training/Learning, networking, Monitoring progress and impact 2 3 4 5 Re-assessment Every 2, 3 or 5 years Self assessment For planning formal assessment With or without A road map for prequalification of products WHO support through Global Learning Opportunities (GLO) & ROs WHO electronic platform to monitor NRAs informaiton and assessment reports, IDP, training, manufacturers, etc Functional NRA HARMONIZED PROCESS TO STRENGTHEN NRA : THE WHO NRA 5 STEP CAPACITY BUILDING RECOMMENDED PROCESS
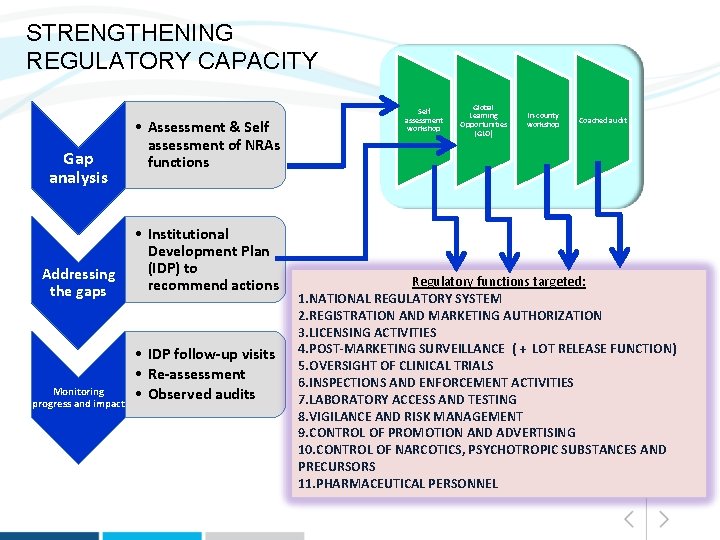
STRENGTHENING REGULATORY CAPACITY Gap analysis • Assessment & Self assessment of NRAs functions • Institutional Development Plan (IDP) to Addressing recommend actions the gaps Monitoring progress and impact • IDP follow-up visits • Re-assessment • Observed audits Self assessment workshop Global Learning Opportunities (GLO) In-county workshop Coached audit Regulatory functions targeted: 1. NATIONAL REGULATORY SYSTEM 2. REGISTRATION AND MARKETING AUTHORIZATION 3. LICENSING ACTIVITIES 4. POST-MARKETING SURVEILLANCE ( + LOT RELEASE FUNCTION) 5. OVERSIGHT OF CLINICAL TRIALS 6. INSPECTIONS AND ENFORCEMENT ACTIVITIES 7. LABORATORY ACCESS AND TESTING 8. VIGILANCE AND RISK MANAGEMENT 9. CONTROL OF PROMOTION AND ADVERTISING 10. CONTROL OF NARCOTICS, PSYCHOTROPIC SUBSTANCES AND PRECURSORS 11. PHARMACEUTICAL PERSONNEL
NEXT STEPS… • Finalized tool by end of sept. 2013 • Computerized tool by Oct. 2013 • Electronic platform for planning and conducting assessment by Oct. 2013 • Tested in Mexico by end of Jan. 2014, then in South Africa (2014), Senegal (2014), Brazil (2014), Viet Nam (2014). • Planning for 2014 -1015 assessment : Nov. 2013 • Integration of Blood and potentially traditional medicines by end of the biennium 2015.
- Slides: 8