THE NUCLEIC ACID Dyah Kinasih Wuragil Veterinary Medicine













- Slides: 51

THE NUCLEIC ACID Dyah Kinasih Wuragil Veterinary Medicine School University of Brawijaya

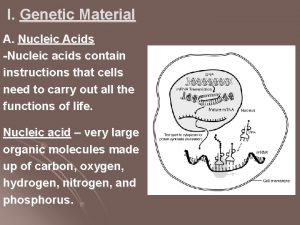
Friedrich Miescher in 1869 • isolated what he called nuclein from the nuclei of pus cells • Nuclein was shown to have acidic properties, hence it became called nucleic acid

Contents 1. 2. 3. 4. Composition of nucleic acids Structure and function of DNA Structures and functions of RNA Properties of nucleic acid

• Section 1 Composition of Nucleic Acid

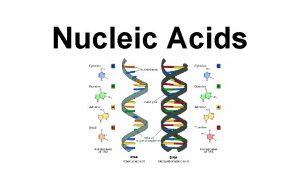
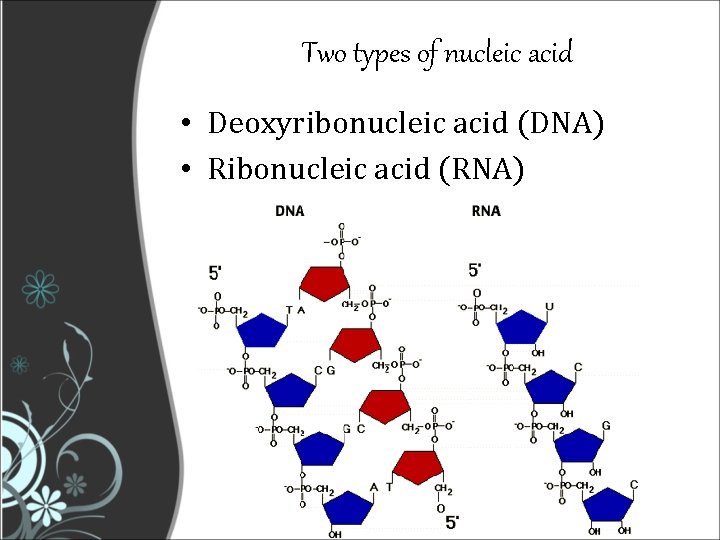
Two types of nucleic acid • Deoxyribonucleic acid (DNA) • Ribonucleic acid (RNA)

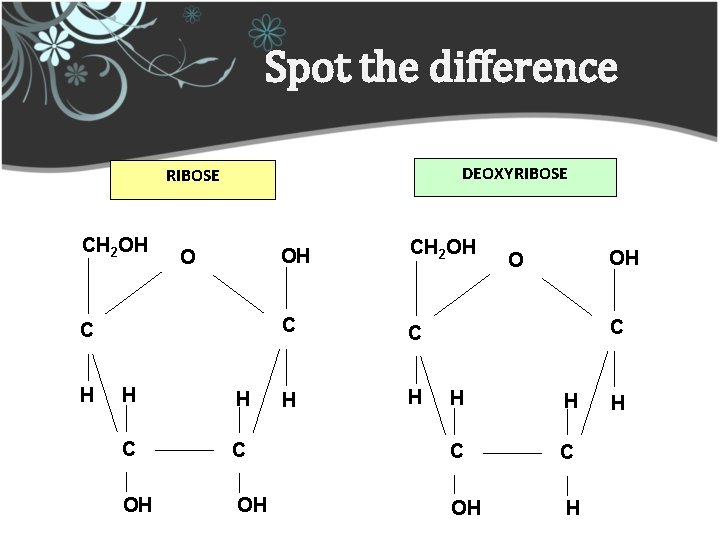
DNA vs RNA • DNA 1 - Deoxyribose sugar 2 - Bases: Adenine, Thymine, Cytosine, Guanine 3 - Double-stranded helix arrangement • RNA 1 - Ribose sugar 2 - Bases: Adenine, Uracyl, Cytosine, Guanine 4 - Single stranded

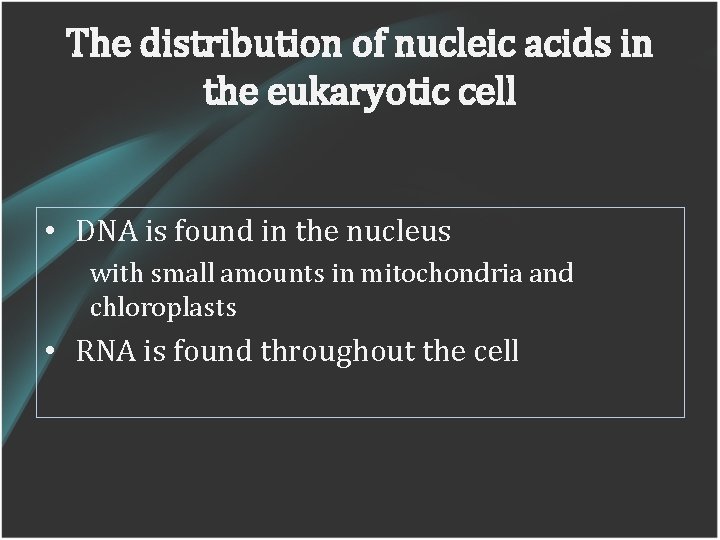
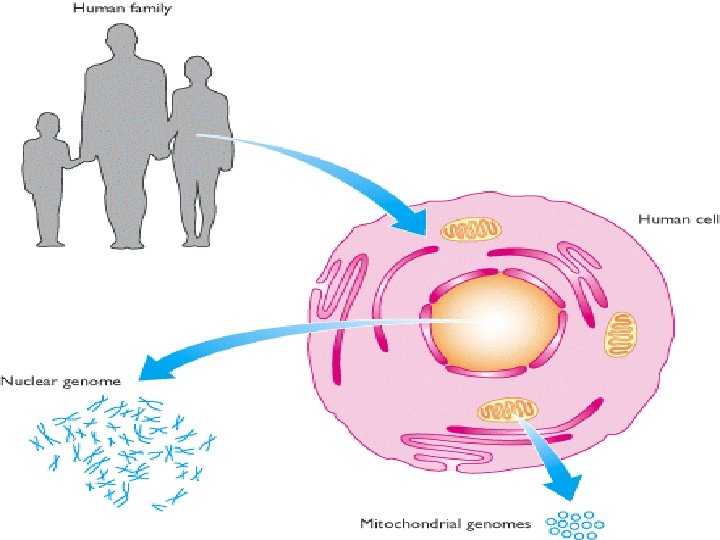
The distribution of nucleic acids in the eukaryotic cell • DNA is found in the nucleus with small amounts in mitochondria and chloroplasts • RNA is found throughout the cell

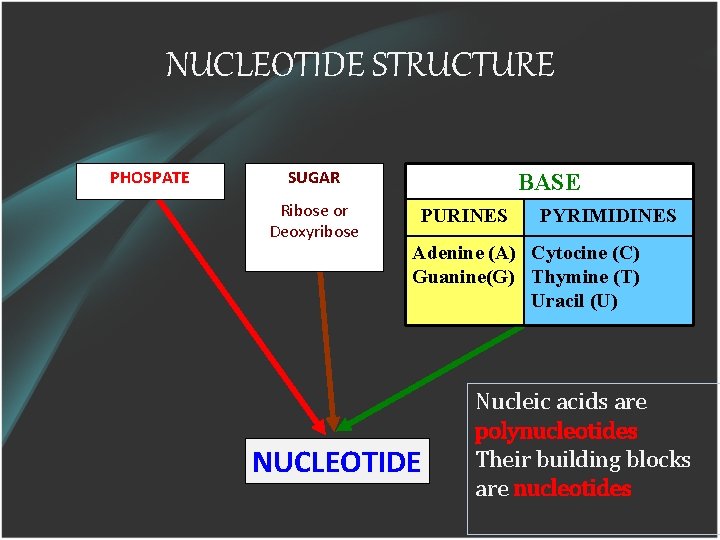
NUCLEOTIDE STRUCTURE PHOSPATE SUGAR Ribose or Deoxyribose BASE PURINES PYRIMIDINES Adenine (A) Cytocine (C) Guanine(G) Thymine (T) Uracil (U) NUCLEOTIDE Nucleic acids are polynucleotides Their building blocks are nucleotides

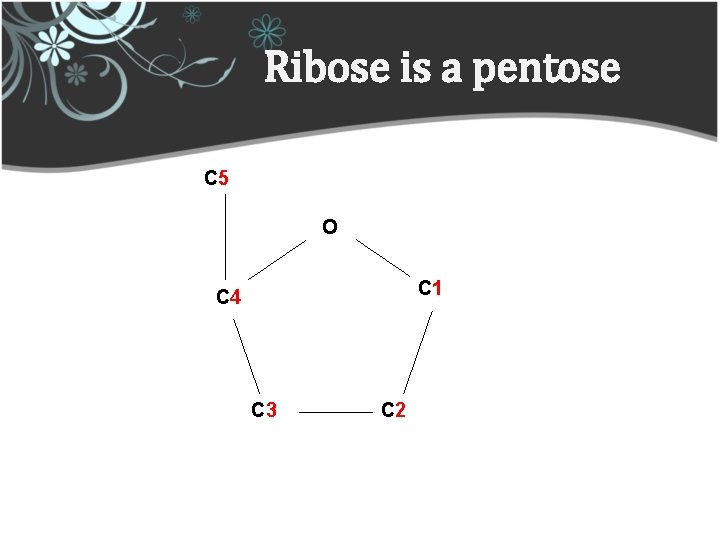
Ribose is a pentose C 5 O C 1 C 4 C 3 C 2

Spot the difference DEOXYRIBOSE CH 2 OH O C H H H C OH OH CH 2 OH C C H H OH O C H H C C C OH OH H H

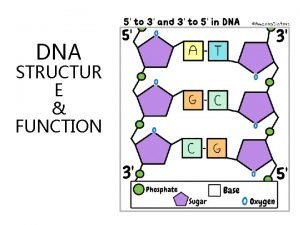
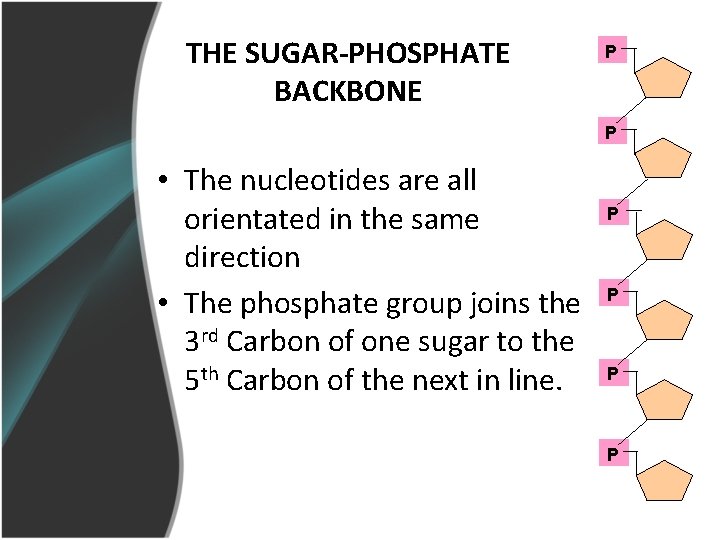
THE SUGAR-PHOSPHATE BACKBONE P P • The nucleotides are all orientated in the same direction • The phosphate group joins the 3 rd Carbon of one sugar to the 5 th Carbon of the next in line. P P

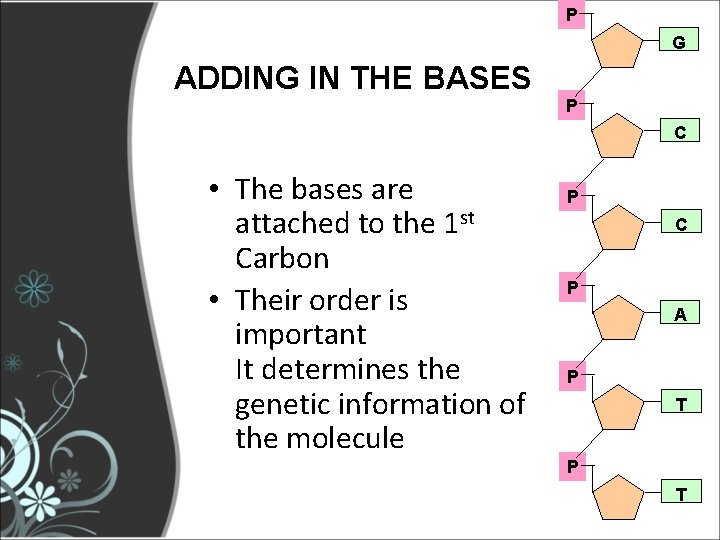
P G ADDING IN THE BASES P C • The bases are attached to the 1 st Carbon • Their order is important It determines the genetic information of the molecule P C P A P T

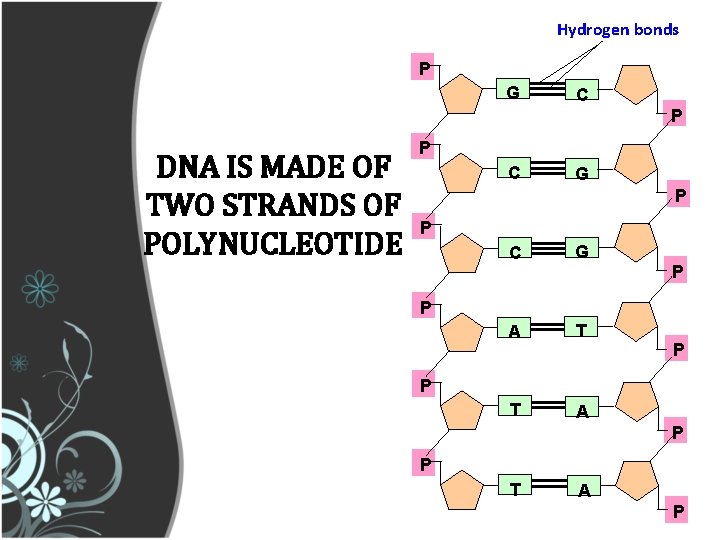
Hydrogen bonds P G C P DNA IS MADE OF TWO STRANDS OF POLYNUCLEOTIDE P C G P P C G A T T A P P P T A P

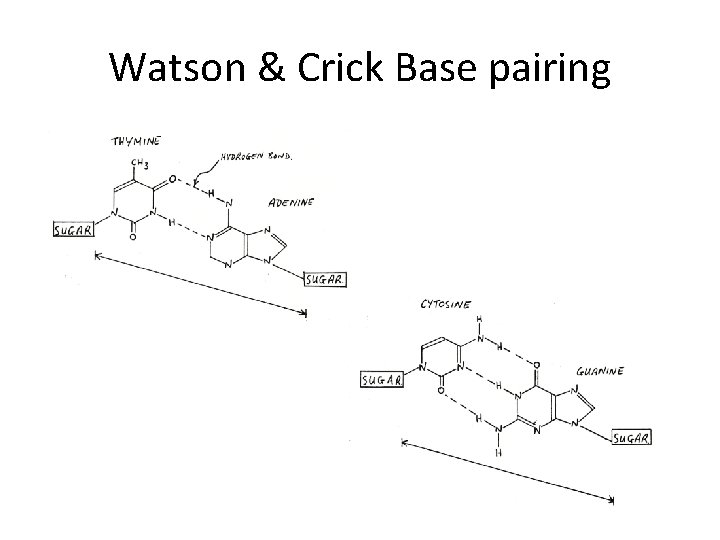
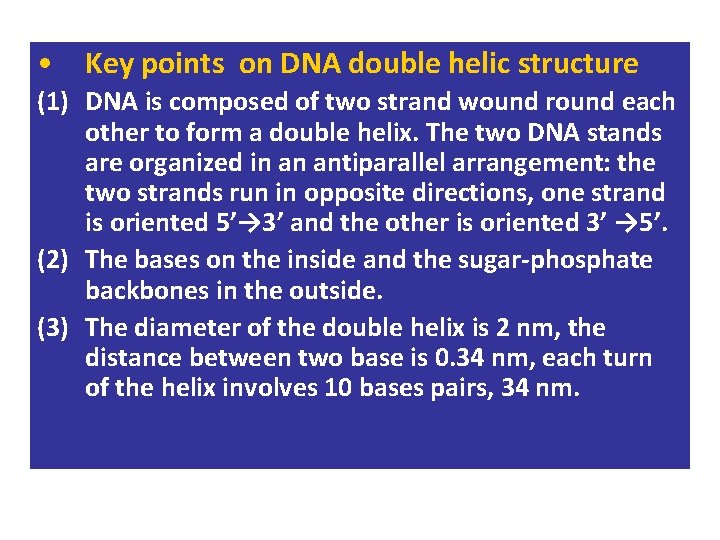
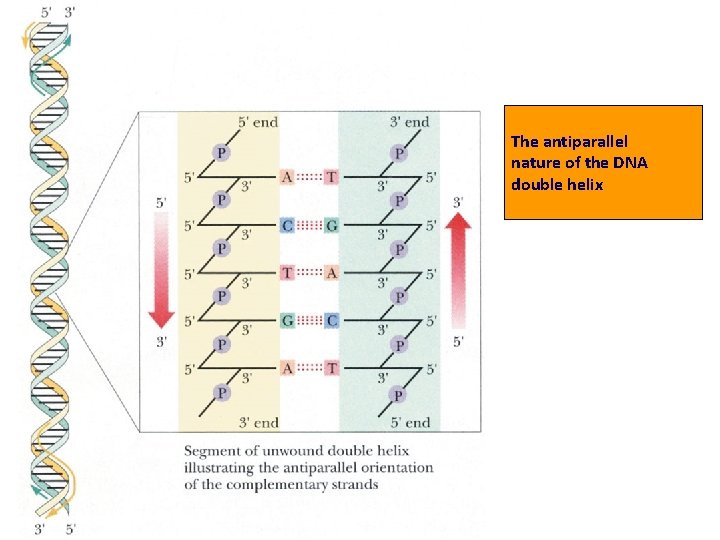
DNA IS MADE OF TWO STRANDS OF POLYNUCLEOTIDE • The sister strands of the DNA molecule run in opposite directions (antiparallel) • They are joined by the bases • Each base is paired with a specific partner: A is always paired with T G is always paired with C Purine with Pyrimidine • This the sister strands are complementary but not identical • The bases are joined by hydrogen bonds, individually weak but collectively strong

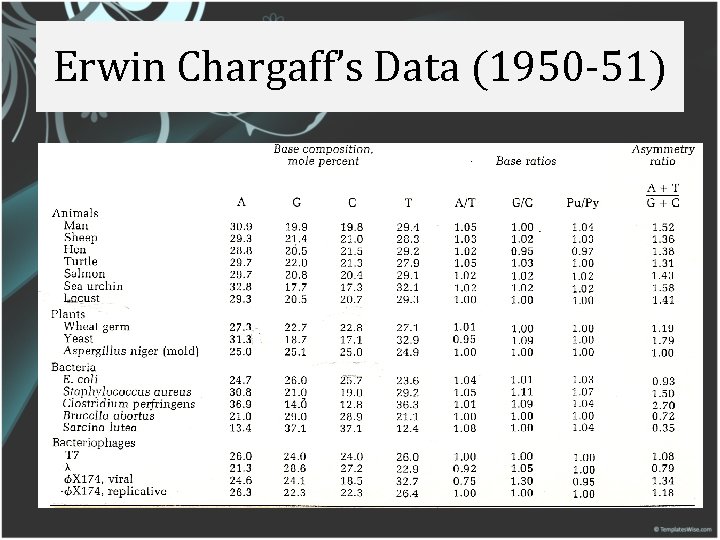
Erwin Chargaff’s Data (1950 -51)

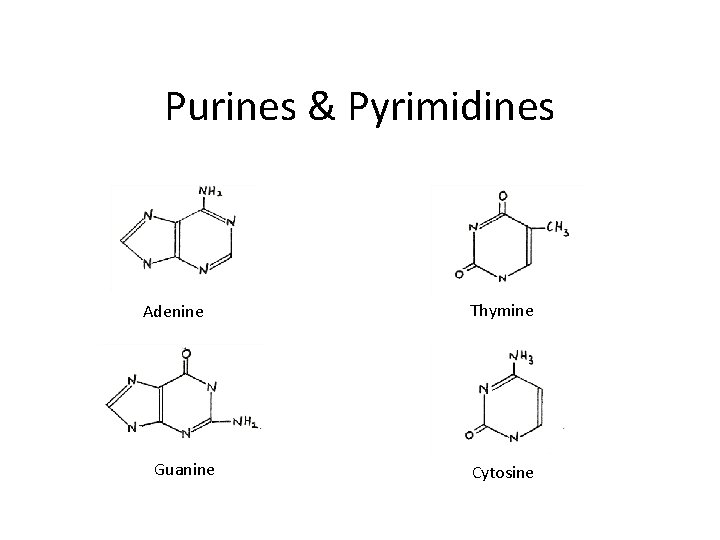
Purines & Pyrimidines Adenine Guanine Thymine Cytosine

Watson & Crick Base pairing

The Double Helix (1953) © Dr Kalju Kahn USBC Chemistry and Biochemistry Public Domain image

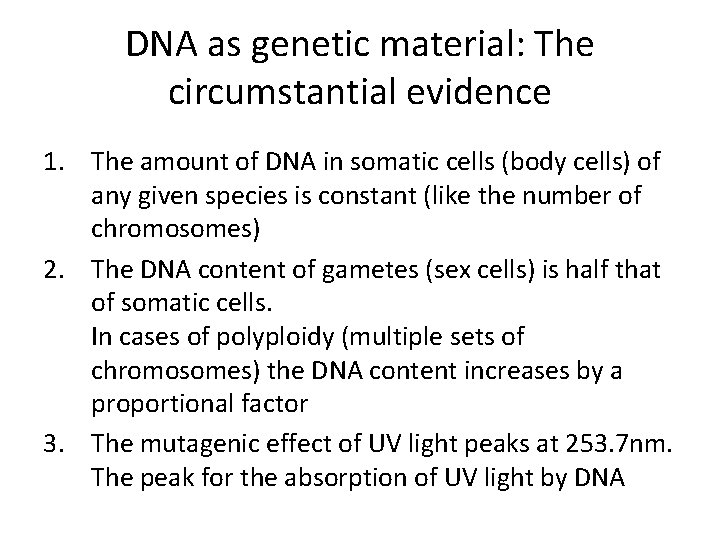
DNA as genetic material: The circumstantial evidence 1. The amount of DNA in somatic cells (body cells) of any given species is constant (like the number of chromosomes) 2. The DNA content of gametes (sex cells) is half that of somatic cells. In cases of polyploidy (multiple sets of chromosomes) the DNA content increases by a proportional factor 3. The mutagenic effect of UV light peaks at 253. 7 nm. The peak for the absorption of UV light by DNA

Some important nucleotides • d. ATP, d. GTP, d. CTP, d. UTP—raw materials for DNA biosynthesis DNA • ATP, GTP, CTP, GTP (1) raw materials for RNA biosynthesis RNA (2) energy donor (3) Important co-enzymes • Cycling nucleotides—c. AMP, c. GMP – secondary messengers in hormones action.

• Section 2 Structure and function of DNA

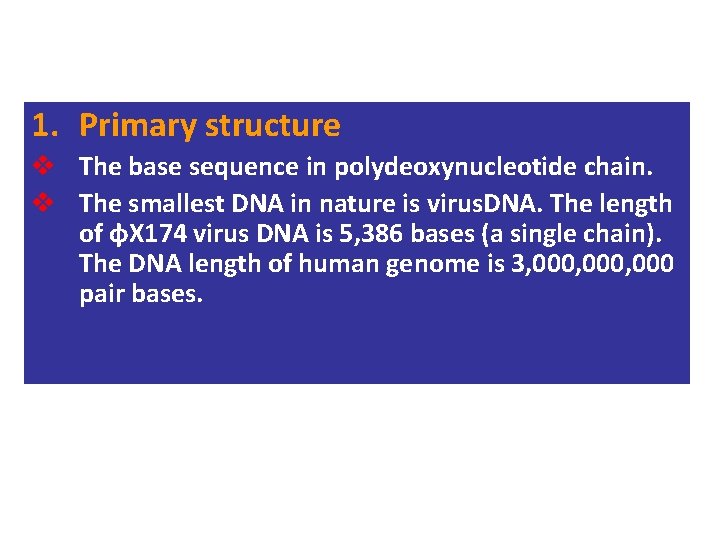
1. Primary structure v The base sequence in polydeoxynucleotide chain. v The smallest DNA in nature is virus. DNA. The length of φX 174 virus DNA is 5, 386 bases (a single chain). The DNA length of human genome is 3, 000, 000 pair bases.

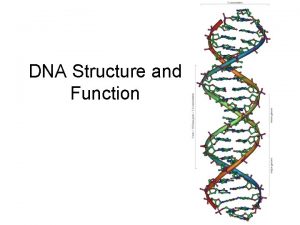
• 2. Secondary structure DNA double helix structure

Francis H. C. Crick James D. Watson

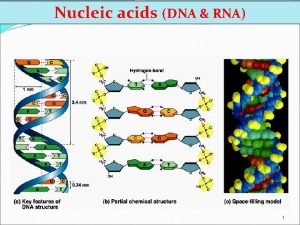
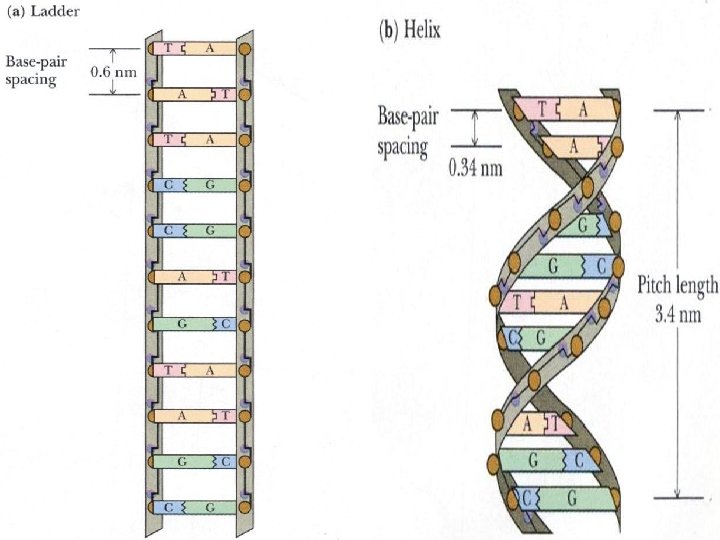
• Key points on DNA double helic structure (1) DNA is composed of two strand wound round each other to form a double helix. The two DNA stands are organized in an antiparallel arrangement: the two strands run in opposite directions, one strand is oriented 5’→ 3’ and the other is oriented 3’ → 5’. (2) The bases on the inside and the sugar-phosphate backbones in the outside. (3) The diameter of the double helix is 2 nm, the distance between two base is 0. 34 nm, each turn of the helix involves 10 bases pairs, 34 nm.

The bases of two strands form hydrogen bonds to each other, A pairs with T, G pairs with C. this is called complementary base pairing • (4) • (5) stable configuration can be maintained by hydrogen bond and base stacking force.

The antiparallel nature of the DNA double helix


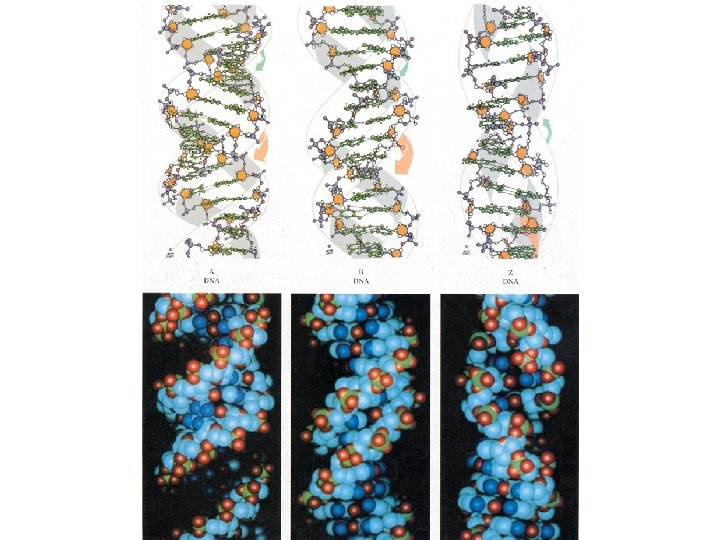
• Conformational variation in double-helical structure • B-DNA • A-DNA • Z-DNA


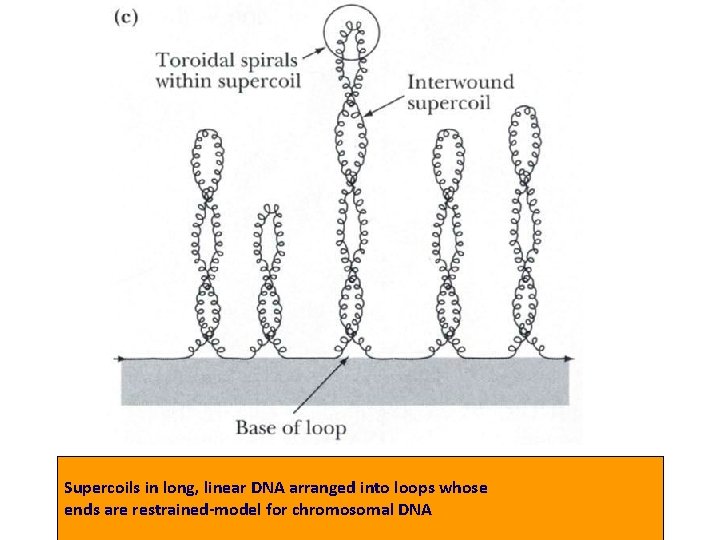
• 3. Tertiary structure : Supercoils: double-stranded circular DNA form supercoils if the strands are underwound (negatively supercoiled) or overwound (positively supercoiled).

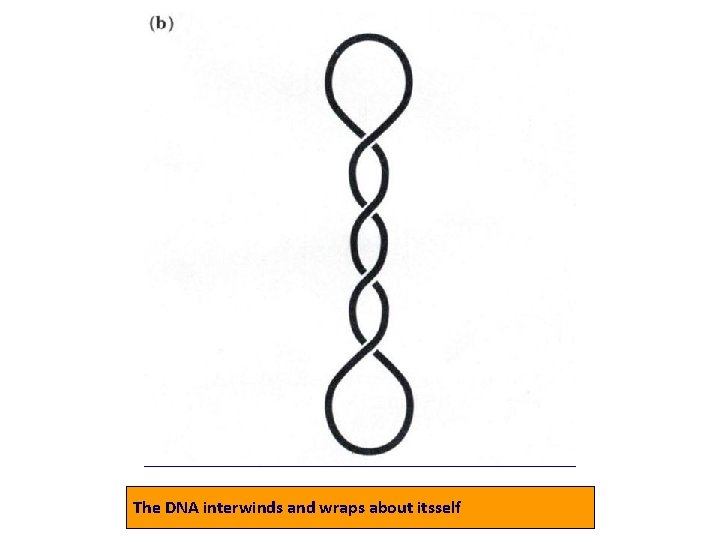
The DNA interwinds and wraps about itsself

Supercoils in long, linear DNA arranged into loops whose ends are restrained-model for chromosomal DNA

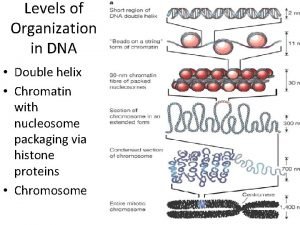
• The DNA in a prokaryotic cell is a supercoil. • The DNA in eukaryotic cell is packaged into chromosomes.

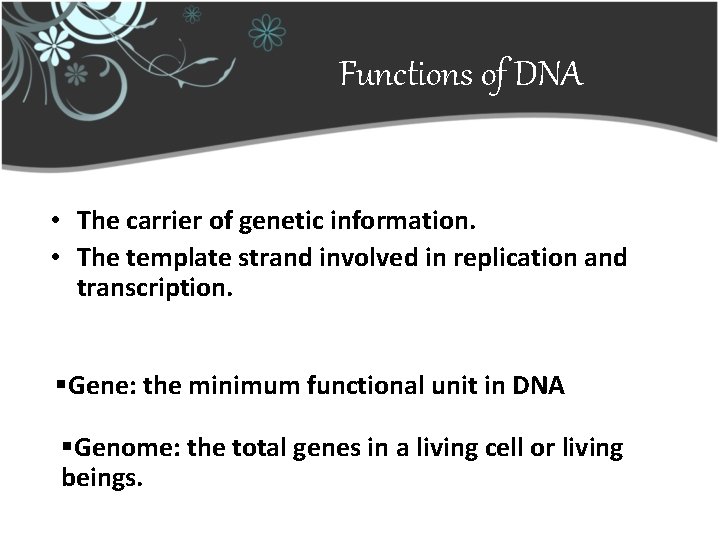
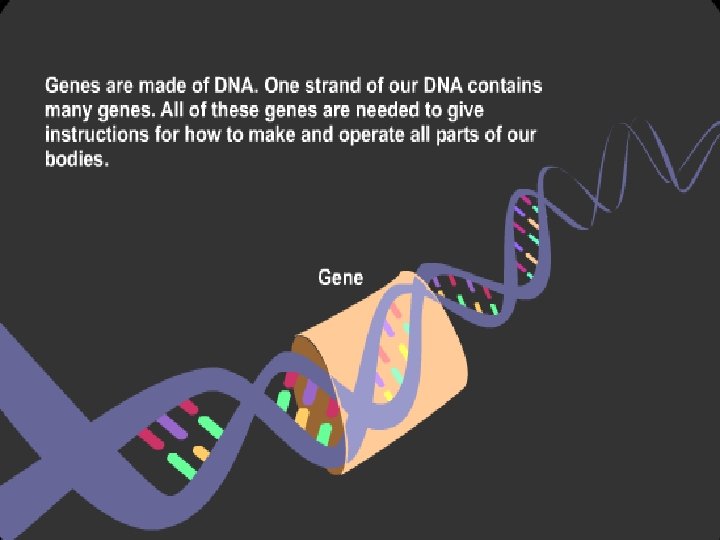
Functions of DNA • The carrier of genetic information. • The template strand involved in replication and transcription. §Gene: the minimum functional unit in DNA §Genome: the total genes in a living cell or living beings.



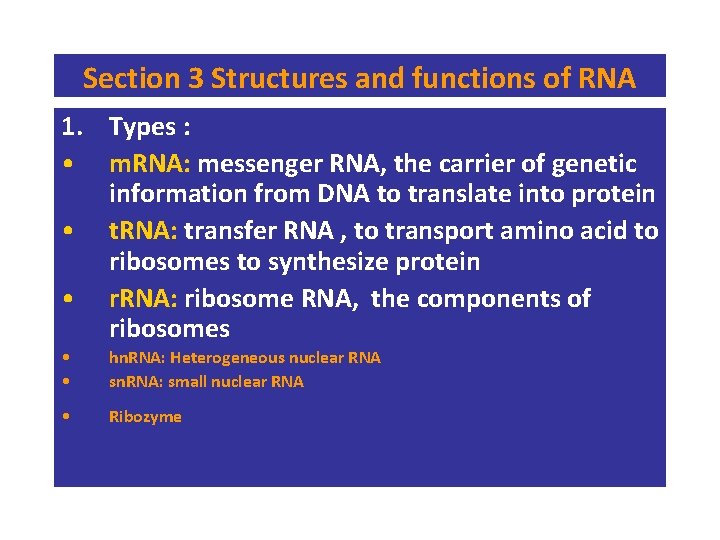
Section 3 Structures and functions of RNA 1. Types : • m. RNA: messenger RNA, the carrier of genetic information from DNA to translate into protein • t. RNA: transfer RNA , to transport amino acid to ribosomes to synthesize protein • r. RNA: ribosome RNA, the components of ribosomes • • hn. RNA: Heterogeneous nuclear RNA sn. RNA: small nuclear RNA • Ribozyme

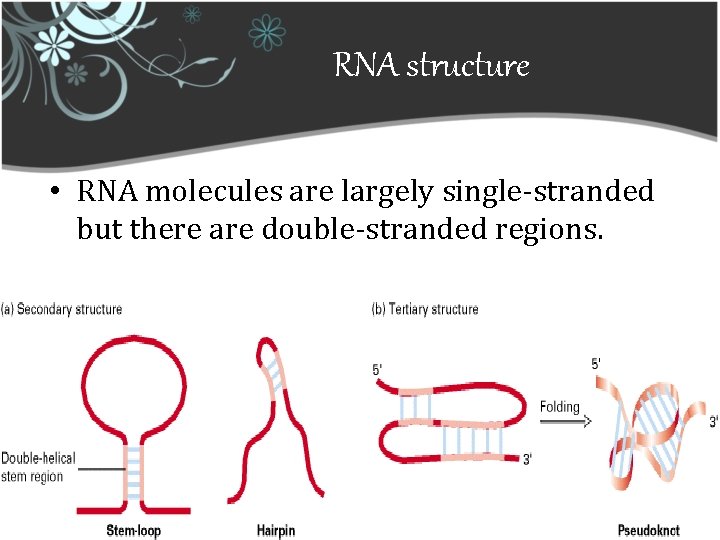
RNA structure • RNA molecules are largely single-stranded but there are double-stranded regions.

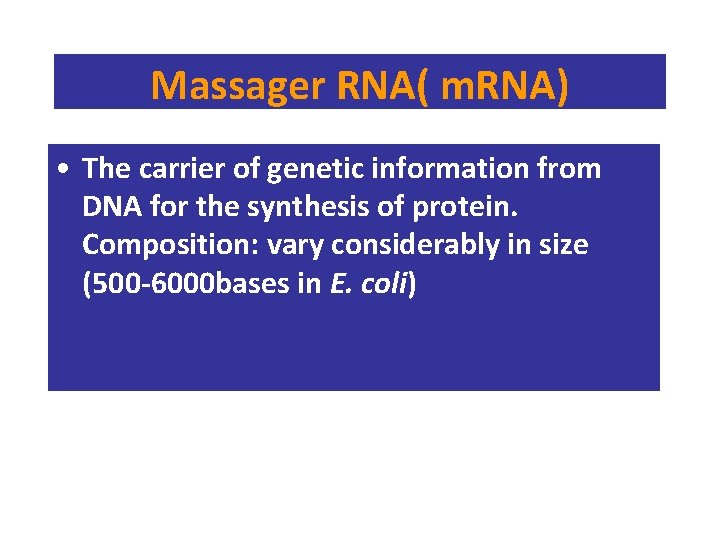
Massager RNA( m. RNA) • The carrier of genetic information from DNA for the synthesis of protein. Composition: vary considerably in size (500 -6000 bases in E. coli)

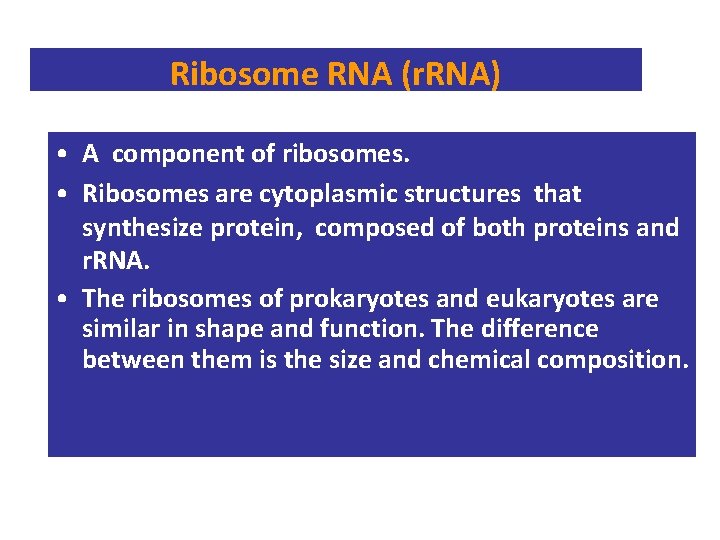
Ribosome RNA (r. RNA) • A component of ribosomes. • Ribosomes are cytoplasmic structures that synthesize protein, composed of both proteins and r. RNA. • The ribosomes of prokaryotes and eukaryotes are similar in shape and function. The difference between them is the size and chemical composition.

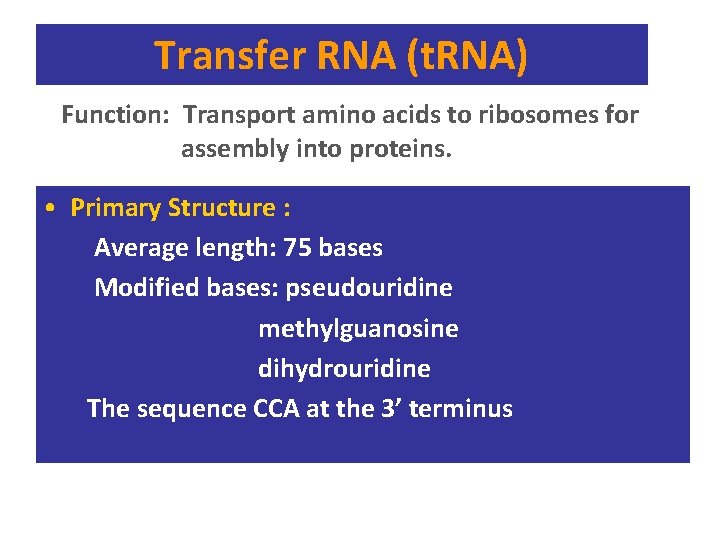
Transfer RNA (t. RNA) Function: Transport amino acids to ribosomes for assembly into proteins. • Primary Structure : Average length: 75 bases Modified bases: pseudouridine methylguanosine dihydrouridine The sequence CCA at the 3’ terminus

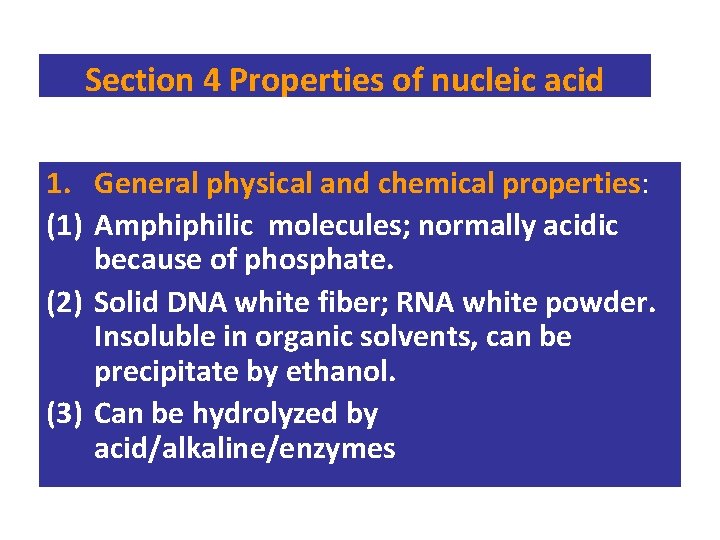
Section 4 Properties of nucleic acid 1. General physical and chemical properties: (1) Amphiphilic molecules; normally acidic because of phosphate. (2) Solid DNA white fiber; RNA white powder. Insoluble in organic solvents, can be precipitate by ethanol. (3) Can be hydrolyzed by acid/alkaline/enzymes

2. UV Absorption • Specific absorption at 260 nm. • This can be used to identify nucleic acid

3. Denaturation • • Concept: the course of hydrogen bonds broken, 3 -D structure was destroyed, the double helix changed into single strand irregular coid • Results: (1) the value of 260 nm absorption is increased (2) Viscous is decreased (3) biological functions are lost

• Heat denaturation and Tm When DNA were heated to certain temperature, the absorption value at 260 nm would increased sharply,which indicates that the double strand helix DNA was separated into single strand. When the absorption value increases to 40%, the value change would low down, which indicates the double strands had been completely separated.

• Tm: melting temperature of DNA • The temperature of UV absorption increase to an half of maximum value in DNA denaturation. • Factors affect Tm: G-C content: there are three hydrogen bonds between G-C pair. The more G-C content, the higher Tm value. • (G+C)% = (Tm-69. 3) × 2. 44

4. Renaturation of DNA • When slowly cooling down the denatured DNA solution, the single strand DNA can reform a double strands helix to recover its biological functions.

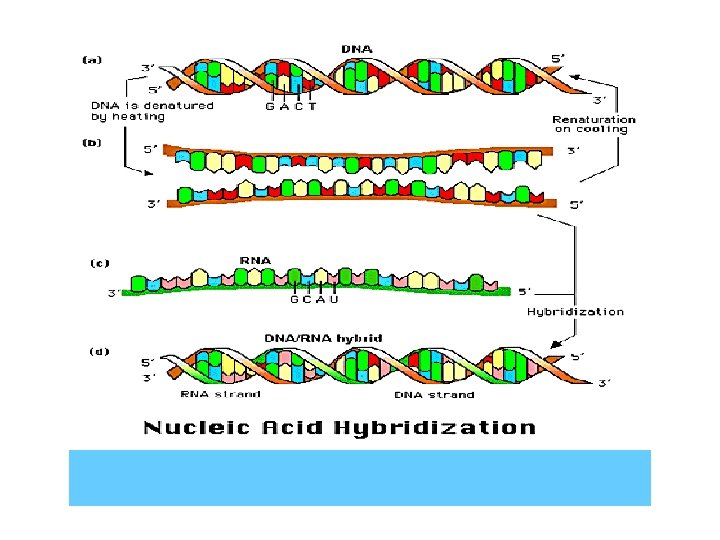
5. Molecule hybridization • During the course of lowing down denaturing temperature, between different resource DNAs or single stand DNA and m. RNA with complementary bases will repair into a double strands to form a hybrid DNA or DNA-RNA. This course is called molecule hybridization.


Thank you. . .
 Kebaikan persero
Kebaikan persero Dyah anggraini gunadarma
Dyah anggraini gunadarma Ika dyah saraswati
Ika dyah saraswati Dna rna
Dna rna Chargaff rule
Chargaff rule Building block of nucleic acid
Building block of nucleic acid Nucleic acid test
Nucleic acid test Nucleic acid
Nucleic acid Dideoxyribonucleic
Dideoxyribonucleic Nucleic acid structure
Nucleic acid structure Dna and rna
Dna and rna Nucleic acid chart
Nucleic acid chart Nucleic acid
Nucleic acid Food rich in nucleic acid
Food rich in nucleic acid Infectious nucleic acid
Infectious nucleic acid Function of nucleic acids
Function of nucleic acids Gastric glands
Gastric glands Nucleic acid
Nucleic acid Purpose of nucleic acid
Purpose of nucleic acid Quinolones mode of action
Quinolones mode of action Nature of nucleic acid
Nature of nucleic acid Nucleic acid made up of
Nucleic acid made up of Nucleic acid dna structure
Nucleic acid dna structure Nucleic acid
Nucleic acid Colony hybridization
Colony hybridization Types of nucleic acid
Types of nucleic acid Dna structure labeled diagram
Dna structure labeled diagram Nucleic acid monomer
Nucleic acid monomer Polymer structure of nucleic acids
Polymer structure of nucleic acids Nucleic acid test
Nucleic acid test Importance of nucleic acid
Importance of nucleic acid Nucleic acid
Nucleic acid Brno veterinary university
Brno veterinary university Faculty of veterinary medicine cairo university
Faculty of veterinary medicine cairo university Dorsocranially
Dorsocranially Veterinary medicine
Veterinary medicine Lincoln memorial university college of veterinary medicine
Lincoln memorial university college of veterinary medicine Vm 544
Vm 544 Cairo university faculty of veterinary medicine
Cairo university faculty of veterinary medicine Faculty of veterinary medicine cairo university logo
Faculty of veterinary medicine cairo university logo Ethics in veterinary medicine
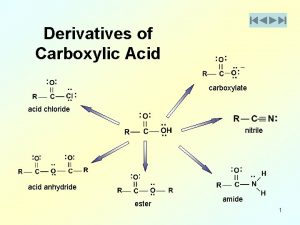
Ethics in veterinary medicine Methanoic anhydride
Methanoic anhydride 9-which acid is not considered a strong acid?
9-which acid is not considered a strong acid? Hcl lewis acid or base
Hcl lewis acid or base Lewis acid vs bronsted acid
Lewis acid vs bronsted acid Non acid fast bacteria
Non acid fast bacteria Acid proton donor or acceptor
Acid proton donor or acceptor Reaction of anhydride with alcohol
Reaction of anhydride with alcohol Lewis acid bronsted acid
Lewis acid bronsted acid Stomach acid vs battery acid
Stomach acid vs battery acid Acid fast and non acid fast bacteria
Acid fast and non acid fast bacteria What are the seven strong acids
What are the seven strong acids