The NOSI an innovative prosthetic treatment option for





- Slides: 21

The NOSI™ : an innovative prosthetic treatment option for nasal speech associated with velopharyngeal dysfunction. Ginette Phippen DClin. P, MRCSLT, Paul Grew MIMP & Mark Richards MIMP The Spires Cleft Centre (Oxford & Salisbury) Salisbury Oral & Maxillofacial Lab Salisbury NHS Foundation Trust

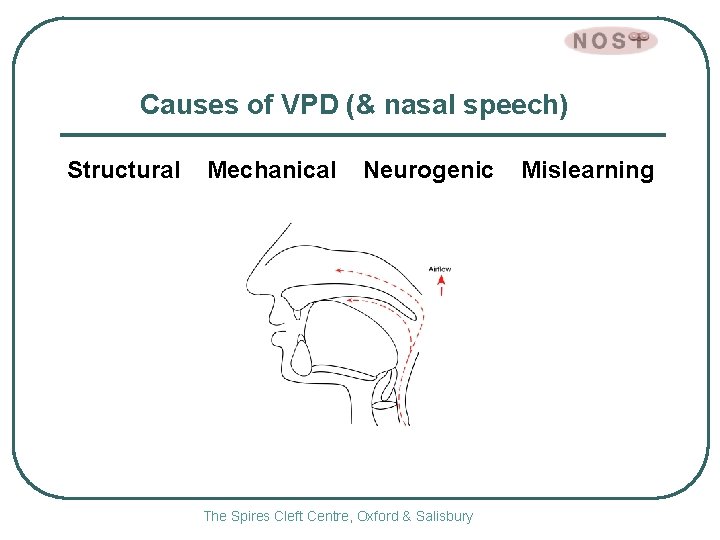
Causes of VPD (& nasal speech) Structural Mechanical Neurogenic The Spires Cleft Centre, Oxford & Salisbury Mislearning

Treatment options l l Surgery (+/- speech therapy) Clear structural defect Speech therapy Mislearned/compensatory sounds l Prosthetics Surgery contraindicated Previous unsuccessful surgery l No intervention l l The Spires Cleft Centre, Oxford & Salisbury

Non-surgical interventions for nasal speech? Palatal prosthetics is an option for some but requires a high level of commitment and resources. Could a nasal obturator offer an immediate, acceptable and low burden option for some patients? The Spires Cleft Centre, Oxford & Salisbury

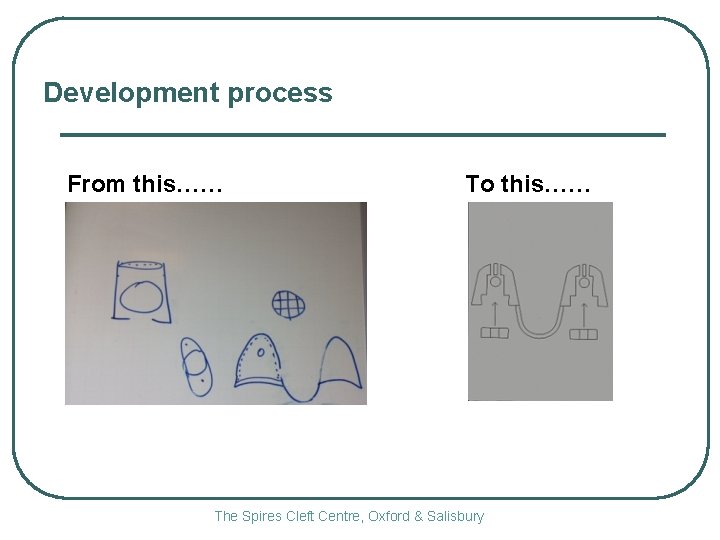
Development process From this…… To this…… The Spires Cleft Centre, Oxford & Salisbury

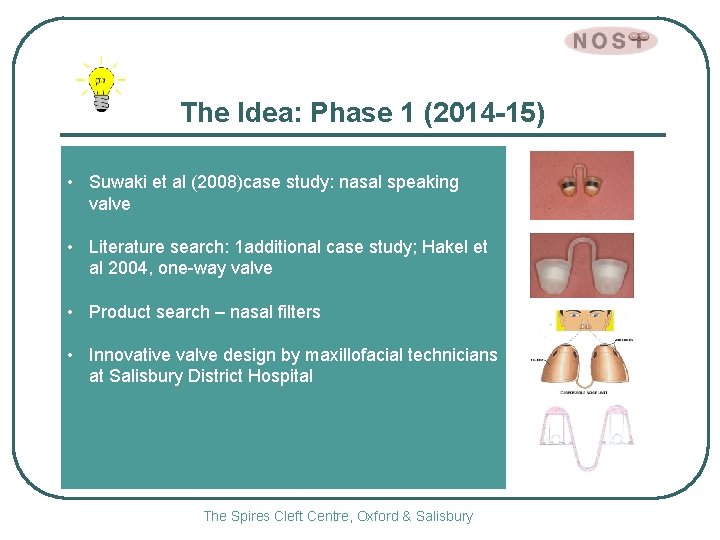
The Idea: Phase 1 (2014 -15) • Suwaki et al (2008)case study: nasal speaking valve • Literature search: 1 additional case study; Hakel et al 2004, one-way valve • Product search – nasal filters • Innovative valve design by maxillofacial technicians at Salisbury District Hospital The Spires Cleft Centre, Oxford & Salisbury

Developing the idea – Phase 1 (2014 -15) • Trademark application/design rights/patenting • 3 d printing • Prosthesis prototypes • Advice from Trust Innovation Lead • Support & Funding from Spires Board • Poster CFSGBI conference (April 2014) • Contact with medical device manufacturers The Spires Cleft Centre, Oxford & Salisbury

Phase 1 Progress Challenges • Positive response to preliminary development from patients and specialist centres • Making progress with development in a busy clinical service • Valuable feedback from patients to inform design • Provision of replacement devices • Support from Innovation Lead • Funding from Spires Charitable Fund for development • Evaluating acceptability and function • Interest from medical device manufacturers • Progressing from prototype to low volume manufacture The Spires Cleft Centre, Oxford & Salisbury

Developing the idea – Phase 2 (2015 -16) • Field testing with 19 patients; including assessment of acceptability and effect on speech • UK cleft centres signed up to test with patients…. . • Small number of devices made at SDH • Potential manufacturer/commercial partner identified The Spires Cleft Centre, Oxford & Salisbury

Prototype field testing Question: Is the NOSI™ acceptable and functional for individuals with nasal speech associated with VPD? The Spires Cleft Centre, Oxford & Salisbury

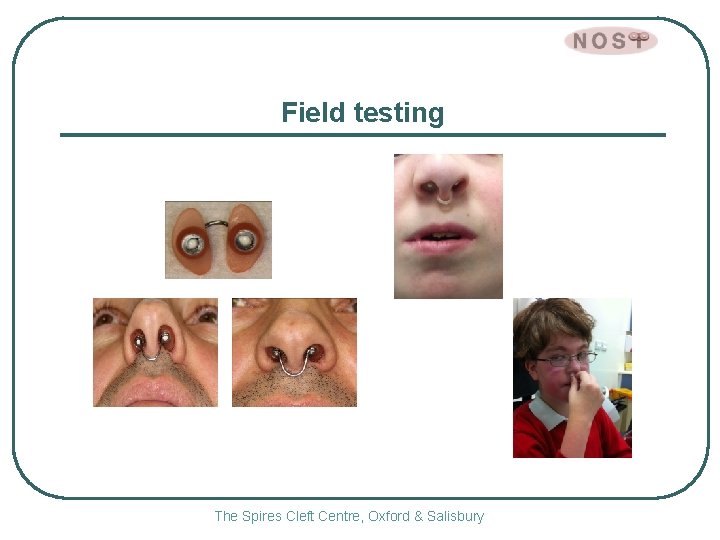
Field testing The Spires Cleft Centre, Oxford & Salisbury

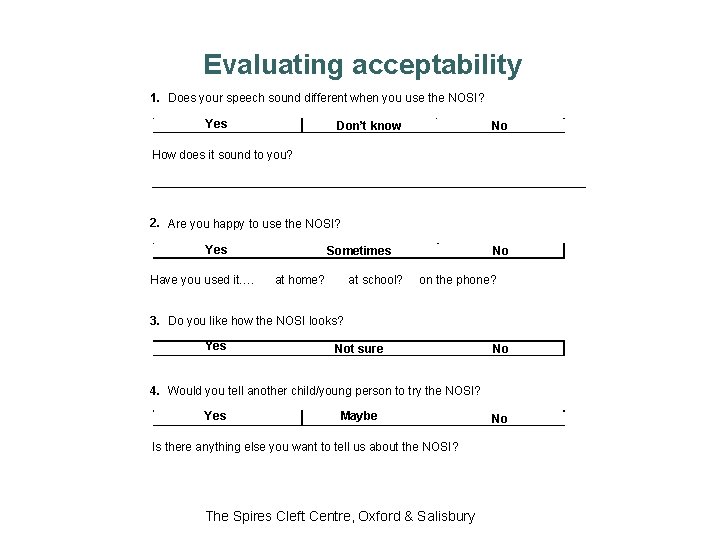
Evaluating acceptability 1. Does your speech sound different when you use the NOSI? Yes Don’t know No How does it sound to you? _________________________________ 2. Are you happy to use the NOSI? Yes Sometimes No Have you used it…. at home? at school? on the phone? 3. Do you like how the NOSI looks? Yes Not sure No 4. Would you tell another child/young person to try the NOSI? Yes Maybe Is there anything else you want to tell us about the NOSI? The Spires Cleft Centre, Oxford & Salisbury No

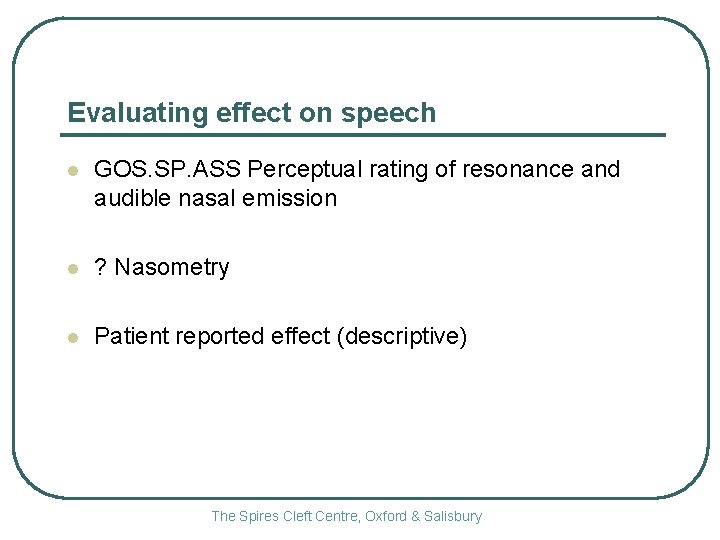
Evaluating effect on speech l GOS. SP. ASS Perceptual rating of resonance and audible nasal emission l ? Nasometry l Patient reported effect (descriptive) The Spires Cleft Centre, Oxford & Salisbury

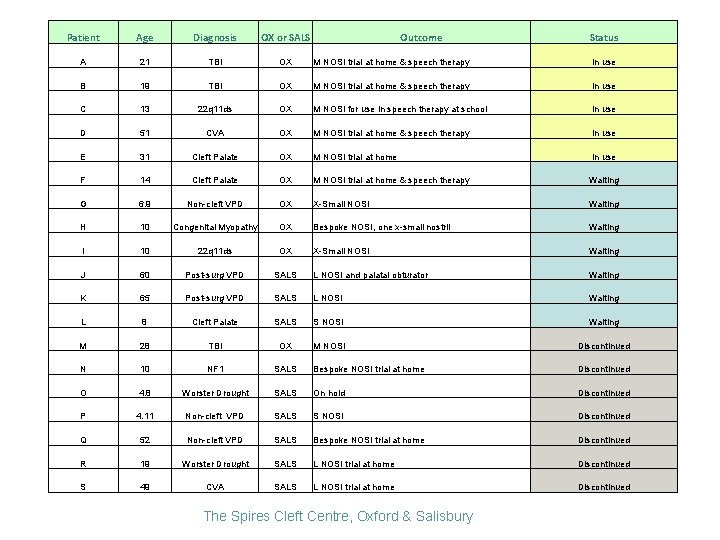
Patient Age Diagnosis OX or SALS Outcome A 21 TBI OX M NOSI trial at home & speech therapy In use B 19 TBI OX M NOSI trial at home & speech therapy In use C 13 22 q 11 ds OX M NOSI for use in speech therapy at school In use D 51 CVA OX M NOSI trial at home & speech therapy In use E 31 Cleft Palate OX M NOSI trial at home In use F 14 Cleft Palate OX M NOSI trial at home & speech therapy Waiting G 6. 9 Non-cleft VPD OX X-Small NOSI Waiting H 10 Congenital Myopathy OX Bespoke NOSI, one x-small nostril Waiting I 10 22 q 11 ds OX X-Small NOSI Waiting J 60 Post-surg VPD SALS L NOSI and palatal obturator Waiting K 65 Post-surg VPD SALS L NOSI Waiting L 8 Cleft Palate SALS S NOSI Waiting M 28 TBI OX M NOSI Discontinued N 10 NF 1 SALS Bespoke NOSI trial at home Discontinued O 4. 8 Worster Drought SALS On hold Discontinued P 4. 11 Non-cleft VPD SALS S NOSI Discontinued Q 52 Non-cleft VPD SALS Bespoke NOSI trial at home Discontinued R 19 Worster Drought SALS L NOSI trial at home Discontinued S 49 CVA SALS L NOSI trial at home Discontinued The Spires Cleft Centre, Oxford & Salisbury Status

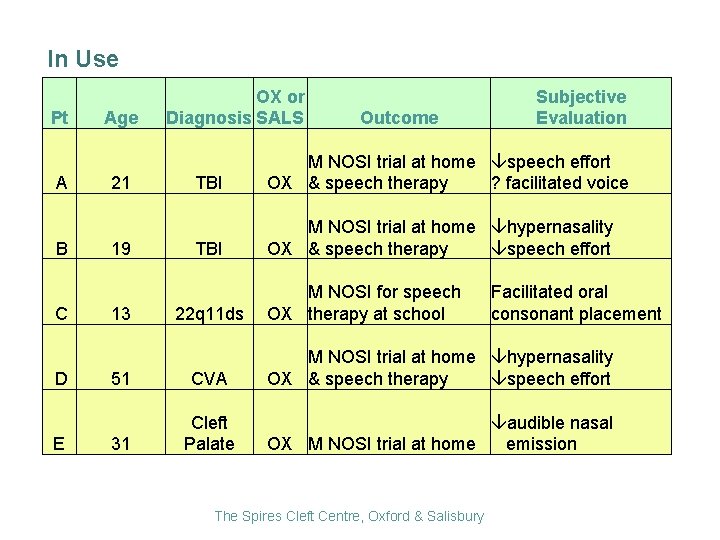
In Use Pt A B C D E Age 21 19 13 OX or Diagnosis SALS Outcome Subjective Evaluation TBI M NOSI trial at home speech effort ? facilitated voice OX & speech therapy TBI M NOSI trial at home hypernasality speech effort OX & speech therapy 22 q 11 ds 51 CVA 31 Cleft Palate M NOSI for speech OX therapy at school Facilitated oral consonant placement M NOSI trial at home hypernasality speech effort OX & speech therapy audible nasal OX M NOSI trial at home emission The Spires Cleft Centre, Oxford & Salisbury

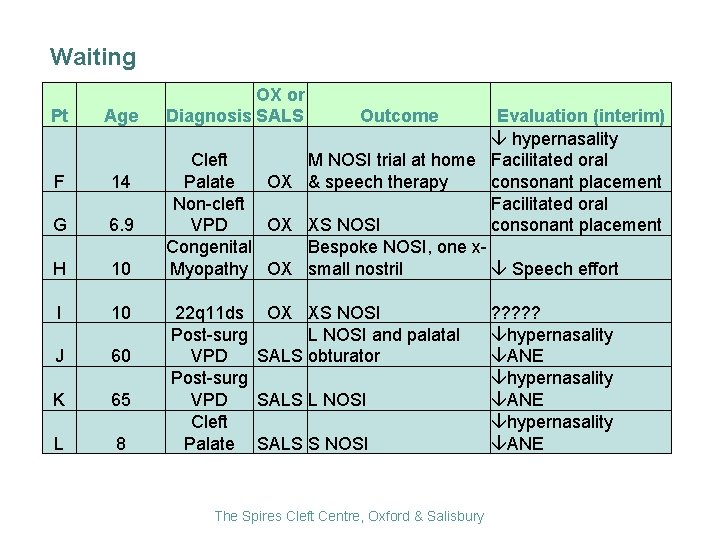
Waiting Pt Age F 14 G 6. 9 H 10 I 10 J 60 K 65 L 8 OX or Diagnosis SALS Outcome Evaluation (interim) hypernasality Cleft M NOSI trial at home Facilitated oral consonant placement Palate OX & speech therapy Non-cleft Facilitated oral VPD OX XS NOSI consonant placement Congenital Bespoke NOSI, one x Speech effort Myopathy OX small nostril 22 q 11 ds OX XS NOSI Post-surg L NOSI and palatal VPD SALS obturator Post-surg VPD SALS L NOSI Cleft Palate SALS S NOSI The Spires Cleft Centre, Oxford & Salisbury ? ? ? hypernasality ANE

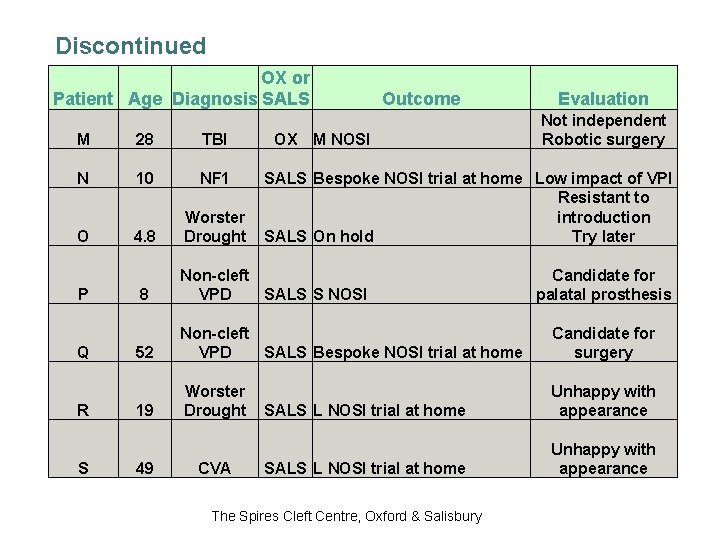
Discontinued OX or Patient Age Diagnosis SALS M 28 TBI N 10 NF 1 4. 8 Worster Drought O P Q R S OX Outcome M NOSI Evaluation Not independent Robotic surgery SALS Bespoke NOSI trial at home Low impact of VPI Resistant to introduction SALS On hold Try later Candidate for palatal prosthesis 8 Non-cleft VPD SALS S NOSI 52 Non-cleft VPD SALS Bespoke NOSI trial at home Candidate for surgery 19 Worster Drought SALS L NOSI trial at home Unhappy with appearance 49 CVA The Spires Cleft Centre, Oxford & Salisbury

Next steps? The Spires Cleft Centre, Oxford & Salisbury

Development process – Phase 2 (2016) • Medical technology company discussions • Prototype 2 prosthesis completed (Injection moulding) • Further field testing with 12 patients and other cleft centres/SLT depts • Class 1 Medical Device/CE marking • Commercialisation process • Formal evaluation The Spires Cleft Centre, Oxford & Salisbury

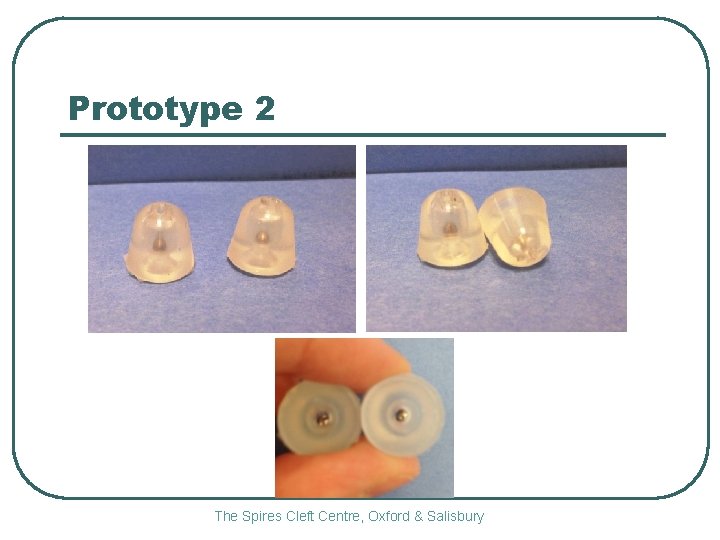
Prototype 2 The Spires Cleft Centre, Oxford & Salisbury

Thank you REFERENCES Suwaki, M. , Nanba, K. , Ito, E. , Kumakura, I. , Minagi, S. (2008) The effect of nasal speaking valve on the speech under experimental incompetence condition. Journal of Oral Rehabilitation, May, 35/5, 361 -9 Hakel, M. , Beukelman, D, R. , Fager, S. , Green, J. , Marshall, J. (2004) Nasal obturator for velopharyngeal dysfunction in dysarthria: Technical report on a one-way valve. Journal of Medical Speech-Language Pathology, December, 12/4, 155 -9. For more information please contact: Ginette Phippen gphippen@nhs. net 01722 345571 ©NOSI™ 2014 WWW. SPIRESCLEFTCENTRE. NHS. UK Sell, D. , Harding, A. & Grunwell, P. (1999). GOS. SP. ASS'98: an assessment for speech disorders associated with cleft palate and / or velopharyngeal dysfunction (revised). Int. J. Language & Communication Disorders, 34(1), 17 -33. The Spires Cleft Centre, Oxford & Salisbury