The NIH Stroke Net Steering Committee Meeting April







































- Slides: 59

The NIH Stroke. Net Steering Committee Meeting April 10, 2019 12: 00 -3: 00 pm ET

Steering Committee Welcome • Welcome and overview - Dr. Broderick • NINDS update and report - Dr. Janis

Recruitment in Prevention Trials – Best Practices • United • University of Iowa • UPMC • Intercoastal

Study Recruitment Tips Heena Olalde, RN, MSN Enrique Leira, MD, MS University of Iowa RCC

Our Experience • Screen inpatient/outpatient stroke service daily • Team approach to recruitment (multiple eyes) • Simple algorithm for eligibility (one source) • Alert clinical team of potential candidate • Involve additional players (ED, transfer center, etc) • Capitalize on EMR resources

Specific Trial Experience • DEFUSE 3 – Utilize Air Care I and II to help with recruitment • CREST 2/H – Delegate to the most promising PI o o o PI = Vascular Surgery Investigators from Vascular Surgery, Neurology, Neurosurgery, Radiology, and Cardiology Coordinators in multiple departments • ARCADIA- Incorporate Prevention Discussion Rounds for any ESUS o o o TOAST classification in EMR Team approach to recruitment Kudos e-mails

Excel in Recruitment • Screen daily for trials • Anticipate and prevent recruitment challenges • Redundant pathways to help with identification and recruitment • Incorporate to patient rounds if possible • Educate your teams about the trials • Explore telehealth, aerial crew, or remote consenting.

Telemedicine Enrollment • Telemedicine allows study personnel to present the informed consent form from a remote location • Requires live two-way video feed via telemedicine and wet ink signature from subject/LAR • Study personnel will arrive on site and be able to immediately begin study interventions

e. Consent Enrollment • e. Consent allows electronic distribution and electronic signature of informed consent form via text or email • Door-to-randomization time reduced • Electronic timestamp may reduce consent documentation errors • Centralized management and monitoring of ICD • Potential to improve participant engagement depending on the study population

e. Consent • Considerations • Is e. Consent appropriate for the trial? • Must be compliant with all relevant regulations, such as the requirements of Part 11 and HIPAA • Cost of implementation

e. Consent • RCC 18 University of Minnesota – experience in prior acute trials • Potential for use in MOST but not currently

Telestroke & e. Consent Christopher Streib, MD, MS Stroke and Telestroke Director, University of Minnesota Abbey Staugaitis, MSN, RN, CCRC U of MN Stroke. Net RCC 18 Program Manager U of MN SIREN HUB Program Manager April 10, 2019

Outline 1. Telestroke Informed Consent at U of MN − − Proposed practice and limitations Future directions 2. Overview of e. Consent 3. Real-world e. Consent experience (HOBIT) − − Features of a good e. Consent platform Lessons learned 4. Conclusions

Abbreviations • e. Consent - electronic consent • SIREN - Strategies to Innovate Eme. Rg. ENcy Care clinical trials network • LAR - legally authorized representative • ICF - informed consent form • e. ICF - electronic informed consent form • HOBIT – Hyperbaric Oxygen Brain Injury Treatment trial

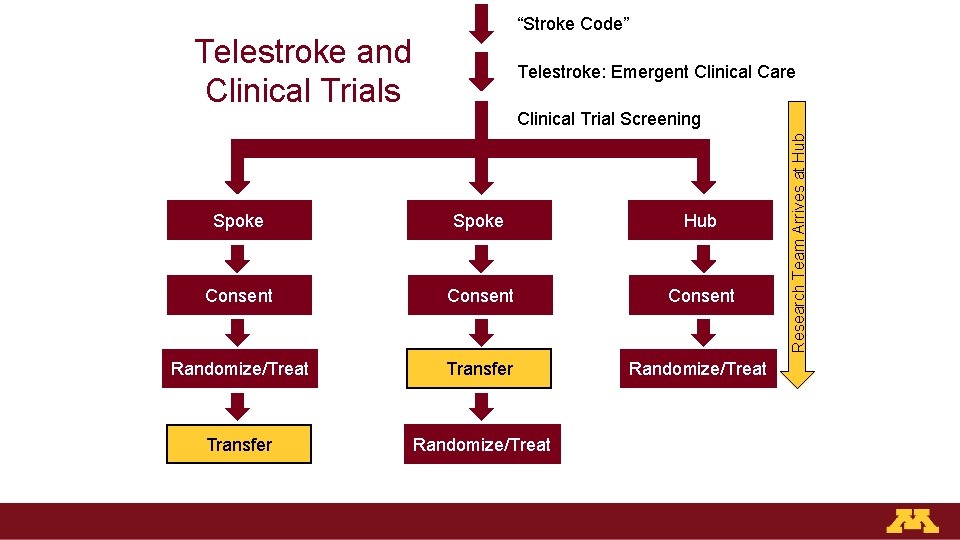
“Stroke Code” Telestroke and Clinical Trials Telestroke: Emergent Clinical Care Spoke Hub Consent Randomize/Treat Transfer Randomize/Treat Research Team Arrives at Hub Clinical Trial Screening

Telestroke and Clinical Trials: Future Directions • U of MN runs “virtual” stroke programs at partner hospitals – Longitudinal inpatient care with 24 -7 telestroke coverage – Transfer rate: 25% • 750+ projected patients in 2019 • Consent, randomization, follow-up would occur remotely Virtual Site Telestroke Clinical Care Screening Consent Randomize/Treat Transfer

Telestroke Research Consent U of MN 1. RN prints ICF from shared drive – RN gives form to patient/LAR 2. Face-to-face informed consent via Telestroke 3. Subject/LAR review ICF, receive own copy 4. Obtain required signatures on the ICF – – Times/dates Ensure ICF reaches hub: screenshot signature page, fax copy, original ICF with patient

Limitations of the Consent Process 1. Printing, copying, faxing are resource intensive and inefficient 2. LAR must be physically present at the spoke hospital with the patient 3. Risk of incorrect version, incomplete, or lost ICFs By addressing these limitations, e. Consent platforms, in combination with robust Telestroke networks, have the potential to modernize and grow stroke clinical research

Electronic Informed Consent • Use of electronic systems and processes…to convey information related to the study and to obtain and document informed consent. 1 • e. Consent streamlines delivery/return and documentation of signed ICF, but does not change the elements of informed consent 1. US Department of Health and Human Services OHRP. Use of Electronic Informed Consent Questions and Answers Guidance for Institutional Review Boards, Investigators, and Sponsors. Dec 2016.

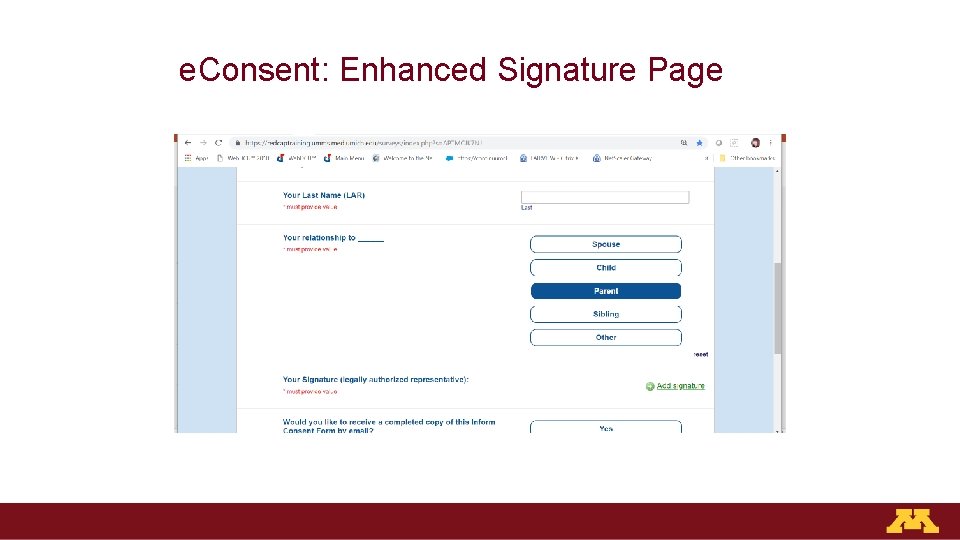
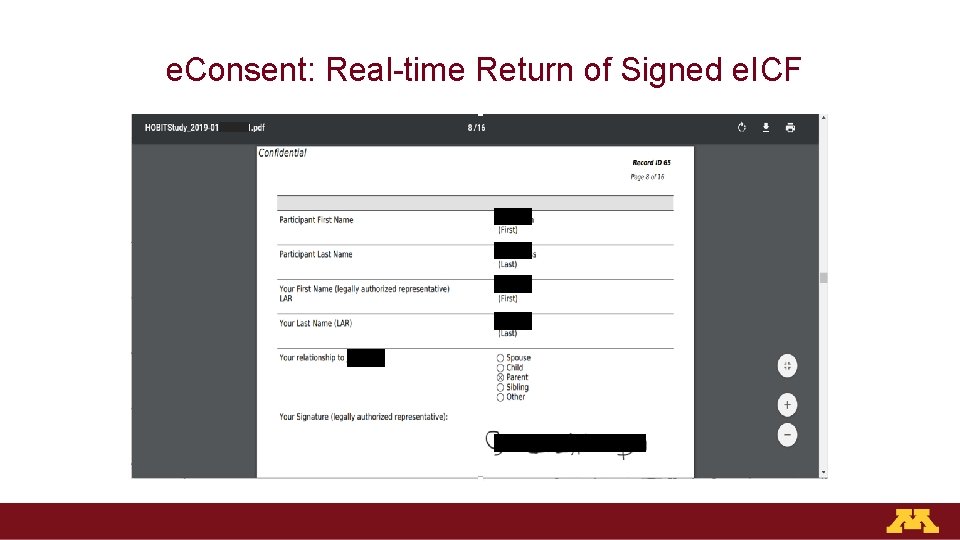
Effective e. Consent Platforms Should Be: 1. Web-based 2. Easy to read and navigate 3. Required elements embedded seamlessly: − Documentation of LAR relationship to participant − LAR must confirm consent conversation occurred and all their questions are answered − Capable of adding capacity questions 4. Real-time return of signed ICF via email to clinicians, research team, LAR/participant

e. ICF: HOBIT

e. ICF: Key Information Highlighted

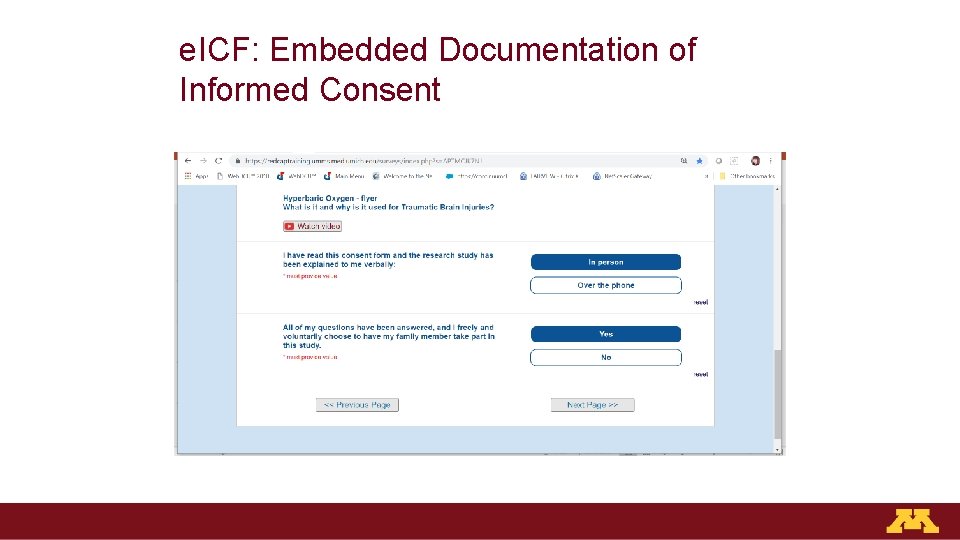
e. ICF: Embedded Documentation of Informed Consent

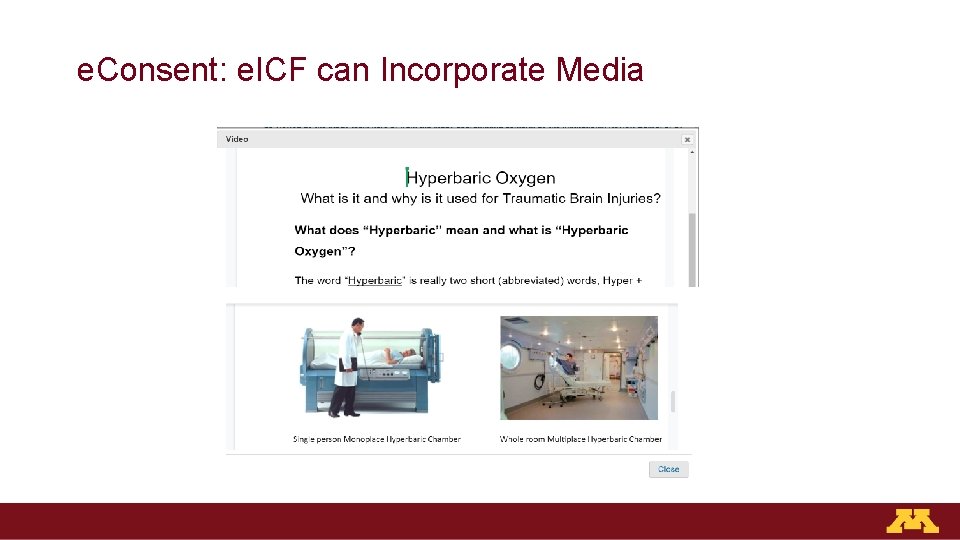
e. Consent: e. ICF can Incorporate Media

e. Consent: Enhanced Signature Page

e. Consent: Real-time Return of Signed e. ICF

e. Consent: Real-time Return of Signed e. ICF

Lessons from HOBIT e. Consent: In Person 1. Test device Wi-Fi connectivity extensively (EDs/MRI/CT) 2. Larger screen preferred 3. Entire clinical team able to navigate e. Consent application smoothly: device location, passwords, bookmarks, links 4. Hard copies of forms may still be necessary

Lessons from HOBIT e. Consent: LAR off-site 1. Clinical team should introduce study 2. Obtain call-back number where LAR can be reliably reached, even while traveling 3. May need multiple phone numbers or email addresses 4. Explain e. Consent process prior to sending link to e. ICF 5. Anticipate follow-up calls

Lessons from HOBIT e. Consent: Telestroke with LAR at Spoke 1. Ensure device connectivity and consistent device storage location − Staff and telestroke team all know location of e. Consent device 2. Investigator team must be able to walk LAR through e. Consent process via Telestroke 3. Current version ICF hard copies available as a back-up

Conclusions 1. Remote/Telestroke research consent is important to optimize stroke trial enrollment 2. Limitations of the current Telestroke consent process can be improved via e. Consent 3. Streamlining informed consent decreases research burden on patient/LAR, research team, and clinicians 4. e. Consent may enhance traditional informed consent 5. A standardized e. Consent platform for Stroke. Net trials should be considered

Thank you • • Chris Streib: streib@umn. edu Abbey Staugaitis: staug 002@umn. edu

Additional Slides

e. Consent Streamlines On & Off-site Consent 1. Delivery of current version of e. ICF directly to patient/LAR 2. e. ICF can be sent to LAR who is not physically present 3. Real time return of signed e. ICF to entire research team/LAR with date and timestamp

e. Consent Enhances Informed Consent 1. More time for informed consent discussion in 2. 3. 4. 5. acute stroke trials Embedded questions in e. Consent document LAR relationship to patient and consent Can embed educational materials (i. e. videos) Real-time access to e. ICFs for study monitors and leadership team Benefits of e. Consent are also applicable when obtaining consent in person

e. Consent Platform Flaws to Avoid 1. Complex end user requirements – Requires subject/LAR to establish an account – Requires the subject or LAR to create a password – Extensive navigation to access consent document 2. Complex research team requirements – Research team creates individual LAR accounts – Complex log-in to access the e. Consent link 3. Data Storage not Part 11 compliant

Exception from Informed Consent (EFIC) • FASTEST Trial • General concept • Will require an entire steering committee future session (s).

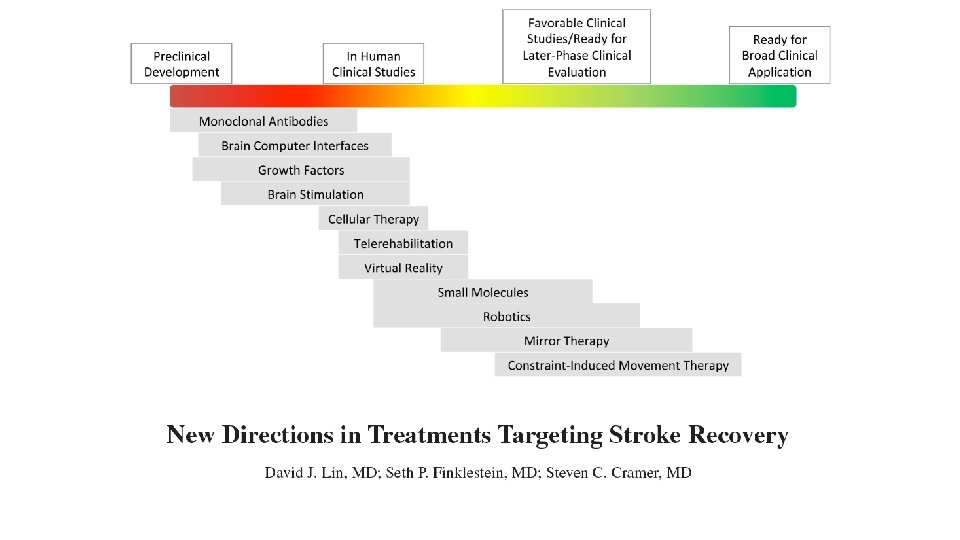
NIH Stroke. Net Recovery & Rehabilitation Group Steven C. Cramer, MD Steven L. Wolf, PT, Ph. D


Stroke. 2018; 49: 3107 -3114

Stroke. 2017; 48: 813 -819.

Stroke. 2017; 48: 813 -819.

Stroke. 2017; 48: 813 -819.

Stroke. 2017; 48: 813 -819.

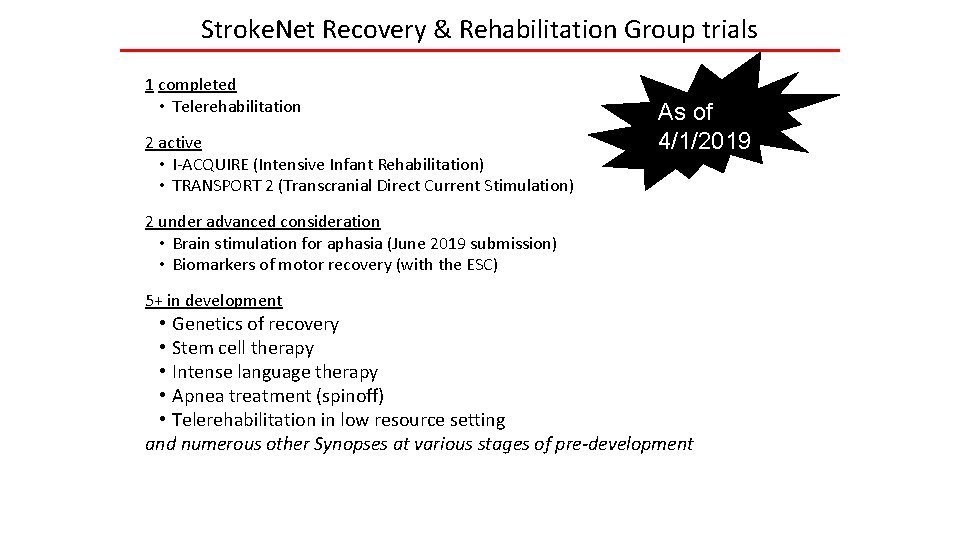
Stroke. Net Recovery & Rehabilitation Group trials 1 completed • Telerehabilitation 2 active • I-ACQUIRE (Intensive Infant Rehabilitation) • TRANSPORT 2 (Transcranial Direct Current Stimulation) As of 4/1/2019 2 under advanced consideration • Brain stimulation for aphasia (June 2019 submission) • Biomarkers of motor recovery (with the ESC) 5+ in development • Genetics of recovery • Stem cell therapy • Intense language therapy • Apnea treatment (spinoff) • Telerehabilitation in low resource setting and numerous other Synopses at various stages of pre-development

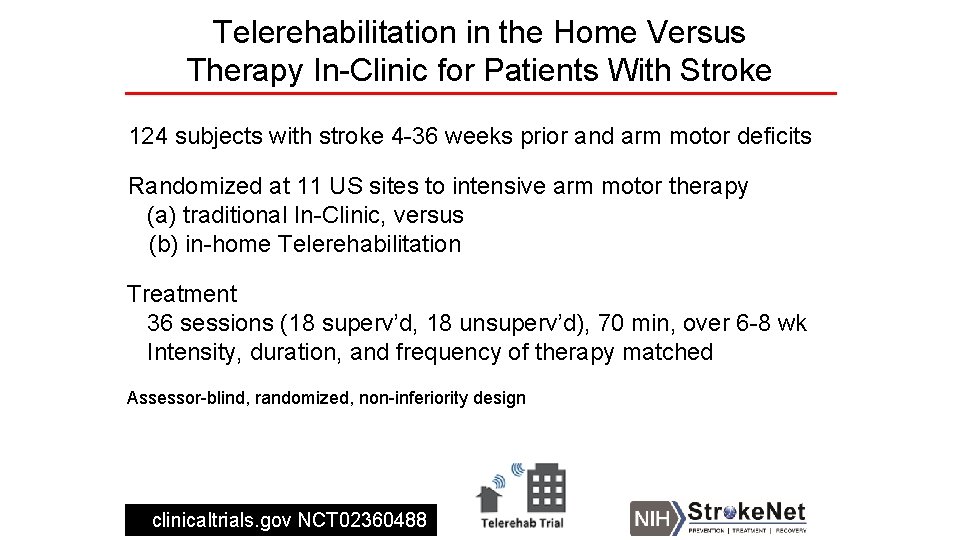
Telerehabilitation In The Home Versus Therapy In-Clinic For Patients With Stroke Steven C. Cramer*, Lucy Dodakian, Vu Le, Jill See, Renee Augsburger, Alison Mc. Kenzie, Robert Zhou, Nina Chiu, Jutta Heckhausen, Jessica M. Cassidy, Walt Scacchi, Megan Therese Smith, A. M. Barrett, Jayme Knutson, Dylan Edwards, David Putrino, Kunal Agrawal, Kenneth Ngo, Elliot J. Roth, David Tirschwell, Michelle L. Woodbury, Ross Zafonte, Wenle Zhao, Judith Spilker, Steven L. Wolf, Joseph P. Broderick, and Scott Janis, for the NIH Stroke. Net Telerehab Investigators *Professor, Depts. Neurology, Anatomy & Neurobiology, and PM&R University of California, Irvine; USA

Telerehabilitation in the Home Versus Therapy In-Clinic for Patients With Stroke 124 subjects with stroke 4 -36 weeks prior and arm motor deficits Randomized at 11 US sites to intensive arm motor therapy (a) traditional In-Clinic, versus (b) in-home Telerehabilitation Treatment 36 sessions (18 superv’d, 18 unsuperv’d), 70 min, over 6 -8 wk Intensity, duration, and frequency of therapy matched Assessor-blind, randomized, non-inferiority design clinicaltrials. gov NCT 02360488

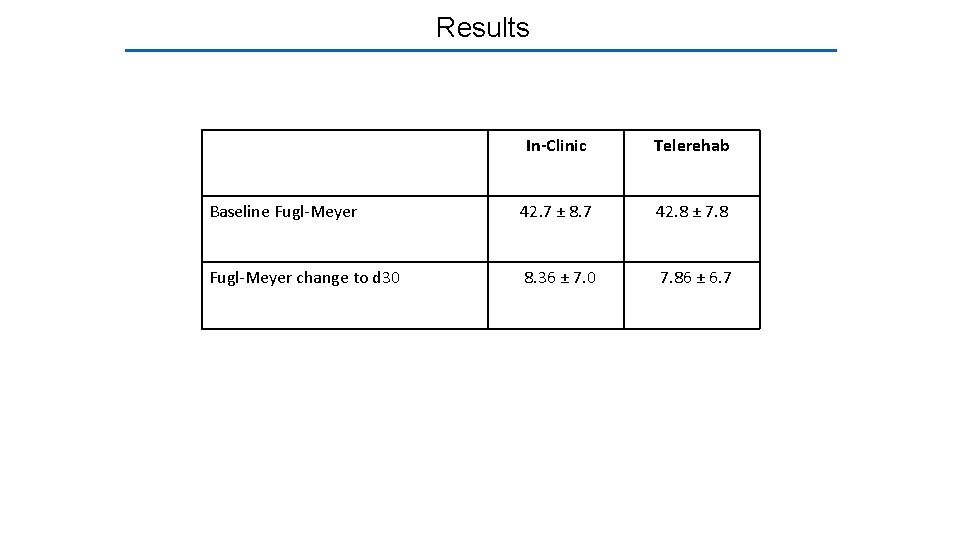
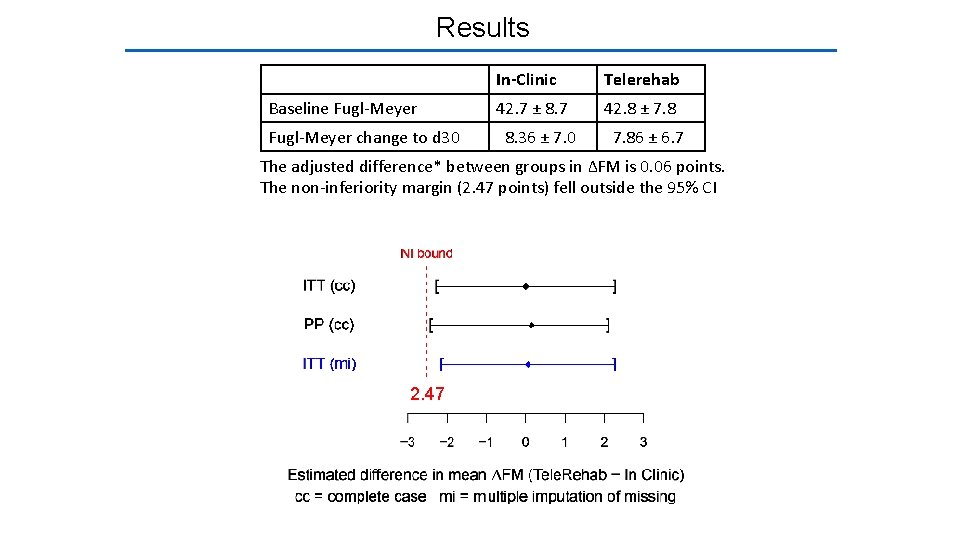
Results In-Clinic Telerehab Baseline Fugl-Meyer 42. 7 ± 8. 7 42. 8 ± 7. 8 Fugl-Meyer change to d 30 8. 36 ± 7. 0 7. 86 ± 6. 7

Results In-Clinic Telerehab Baseline Fugl-Meyer 42. 7 ± 8. 7 42. 8 ± 7. 8 Fugl-Meyer change to d 30 8. 36 ± 7. 0 7. 86 ± 6. 7 The adjusted difference* between groups in ΔFM is 0. 06 points. The non-inferiority margin (2. 47 points) fell outside the 95% CI 2. 47 *Adjusted for age, baseline FM, time post-stroke, enrollment site, and stroke subtype

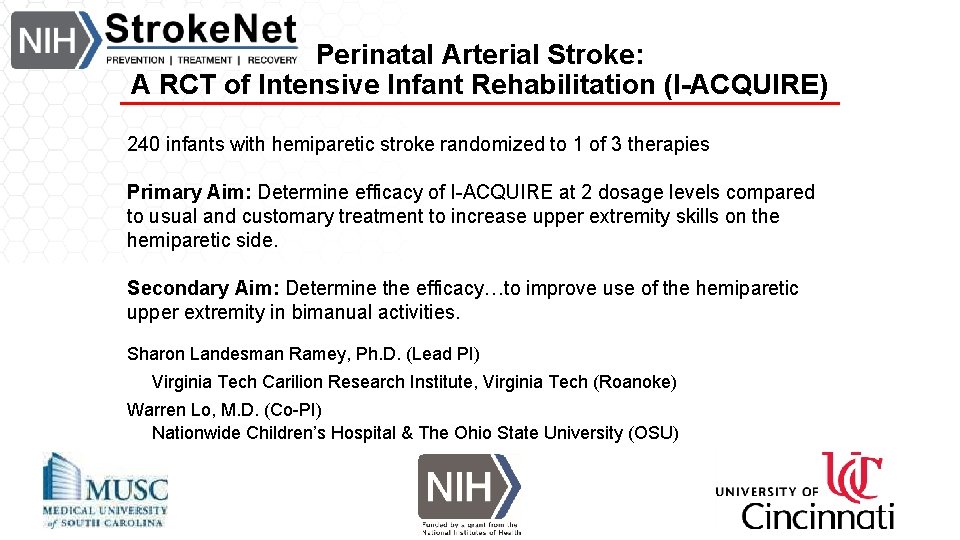
Perinatal Arterial Stroke: A RCT of Intensive Infant Rehabilitation (I-ACQUIRE) 240 infants with hemiparetic stroke randomized to 1 of 3 therapies Primary Aim: Determine efficacy of I-ACQUIRE at 2 dosage levels compared to usual and customary treatment to increase upper extremity skills on the hemiparetic side. Secondary Aim: Determine the efficacy…to improve use of the hemiparetic upper extremity in bimanual activities. Sharon Landesman Ramey, Ph. D. (Lead PI) Virginia Tech Carilion Research Institute, Virginia Tech (Roanoke) Warren Lo, M. D. (Co-PI) Nationwide Children’s Hospital & The Ohio State University (OSU)

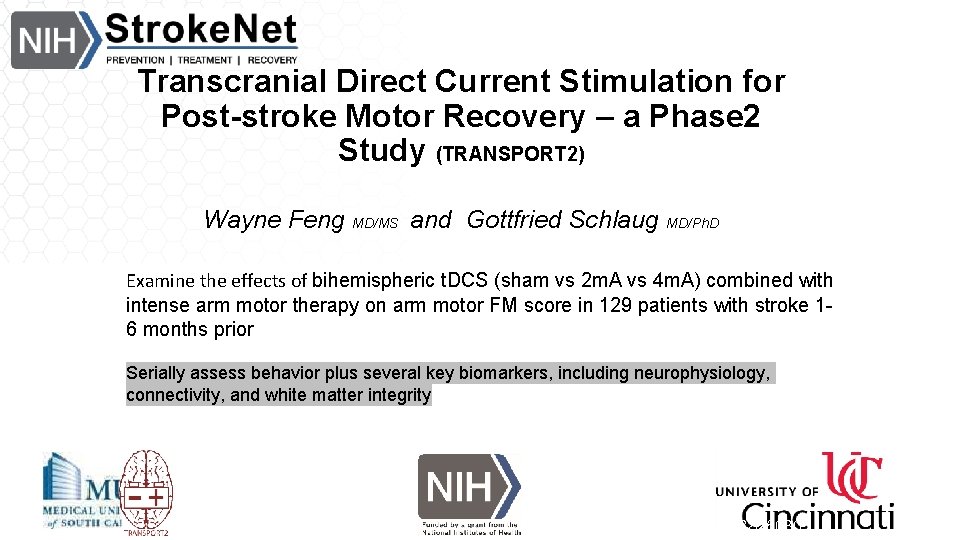
Transcranial Direct Current Stimulation for Post-stroke Motor Recovery – a Phase 2 Study (TRANSPORT 2) Wayne Feng MD/MS and Gottfried Schlaug MD/Ph. D Examine the effects of bihemispheric t. DCS (sham vs 2 m. A vs 4 m. A) combined with intense arm motor therapy on arm motor FM score in 129 patients with stroke 16 months prior Serially assess behavior plus several key biomarkers, including neurophysiology, connectivity, and white matter integrity Clinicaltrials. gov NCT 03826030

NIH Stroke. Net Recovery & Rehabilitation Group We welcome discussions at all stages of Synopsis development We are available to join your RCC meetings, explain, answer Qs Steven C. Cramer, MD Steven L. Wolf, PT, Ph. D Or just email me scramer@uci. edu

The NIH Stroke. Net Central IRB Michael Linke, Ph. D, CIP Chair, CIRB David Ficker, MD, Vice Chair CIRB Susan K. Roll, RN, BSN, CIRB Liaison Jo Ann Behrle, CIRB Coordinator Keeley Hendrix, CIRB Coordinator

Reliance Agreement • Responsibilities of the CIRB • Duties, Rights, and Responsibilities of the Relying Institution (RI)

Reliance Agreement - Basic Responsibilities • CIRB • is the single IRB of record. • regulatory responsibility for assuring the protection of the rights and welfare of research participants. • Relying Institution (RI) • sets standards to determine whether a research investigator can conduct research under its auspices. • agrees to cede IRB review of the NIH Stroke. Net research to the CIRB. • agrees to accept the decisions of the CIRB regarding review, approval and oversight of research covered by this Agreement.

CIRB and MOST/SLEEP SMART Trials • Factors slowing down trial start-up • Lack of knowledge about the NIH Stroke. Net processes at new trial sites • Many sites will not sign CTAs until their site has local and/or CIRB approval • Strategies to facilitate the execution of CTAs • Send a cover letter explaining the process to help expedite the approvals • Also send the approved consent template as a resource

Competing trials/overlap and enrollment strategies • Every Stroke. Net site has to have a locally defined process for competing trials and communicate the plan with the National Coordinating Center. We don’t legislate the approach. • • • Rolling prioritization enrollment grid Equal opportunity enrollment grid Prioritize lagging trials Maximize patient autonomy Minimize Trial Participation

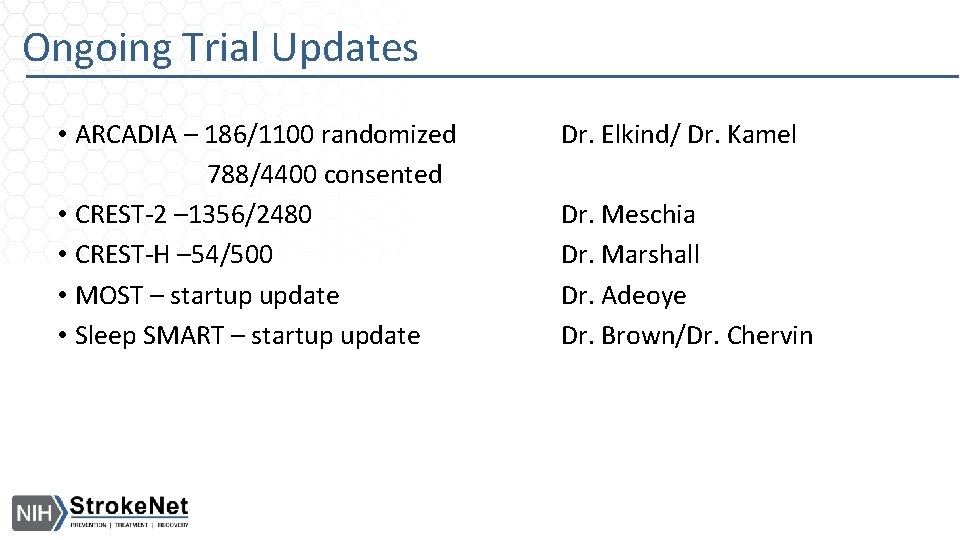
Ongoing Trial Updates • ARCADIA – 186/1100 randomized 788/4400 consented • CREST-2 – 1356/2480 • CREST-H – 54/500 • MOST – startup update • Sleep SMART – startup update Dr. Elkind/ Dr. Kamel Dr. Meschia Dr. Marshall Dr. Adeoye Dr. Brown/Dr. Chervin

Ongoing Trial Updates • TRANSPORT 2 – startup update • I-ACQUIRE – startup update • ASPIRE – pre-award update • SATURN – pre-award update • FASTEST – pre-award update • ARCADIA-CSI – pre-award update Dr. Schlaug/Dr. Feng Dr. Ramey/Dr. Lo Dr. Sheth Dr. Selim Dr. Broderick Dr. Lansberg