The NHLBI Specialized Center of Clinically Oriented Research

The NHLBI Specialized Center of Clinically Oriented Research (SCCOR) in Pediatric Heart Disease at the Children's Hospital of Philadelphia: P 50 -HL 74731 Program Title: Genetic Mechanisms in Pediatric Heart Disease http: //stokes. chop. edu/programs/sccor/ Program Director: Robert J. Levy, M. D. The Joseph Stokes, Jr. , Research Institute

External Advisory Board Elazer R. Edelman, MD, Ph. D, FACC Director, Harvard-MIT Biomedical Engineering Center Thomas D. and Virginia W. Cabot Professor Health Sciences and Technology Massachusetts Institute of Technology & Harvard Medical School, Attending Cardiologist, Brigham and Women’s Hospital David H. Ledbetter, Ph. D. , Robert W. Woodruff Professor of Human Genetics Director, Division of Medical Genetics Emory University School of Medicine Jurg Ott, Ph. D. Professor and Head Laboratory of Statistical Genetics Rockefeller University The Joseph Stokes, Jr. , Research Institute

The CHOP SCCOR—Programmatic Hypothesis “The CHOP SCCOR is a direct outgrowth of productive research at our Institution over the past decade that was based on the hypothesis that congenital heart abnormalities are caused by gene defects” “Basic discoveries concerning gene abnormalities and related patterns of gene expression can be applied to a unifying approach for both understanding the complex basis for cardiac dysmorphogenesis as well as providing therapeutic insights for translational directions. ” The Joseph Stokes, Jr. , Research Institute
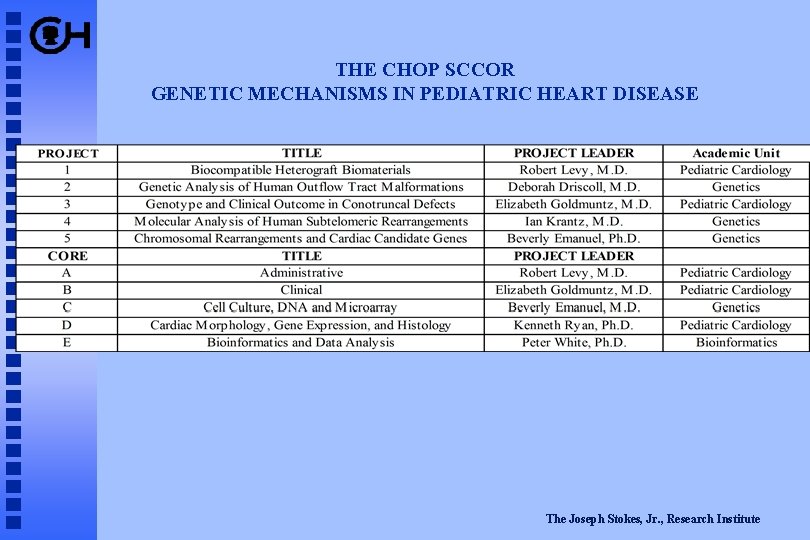
THE CHOP SCCOR GENETIC MECHANISMS IN PEDIATRIC HEART DISEASE The Joseph Stokes, Jr. , Research Institute
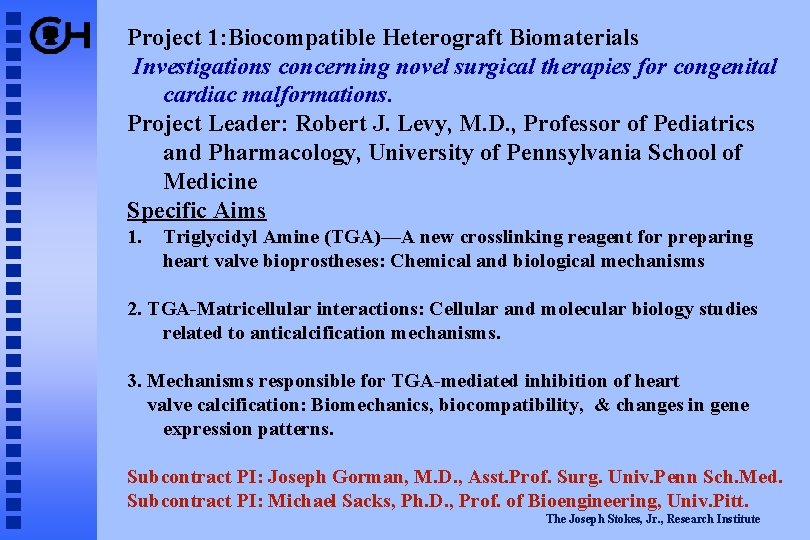
Project 1: Biocompatible Heterograft Biomaterials Investigations concerning novel surgical therapies for congenital cardiac malformations. Project Leader: Robert J. Levy, M. D. , Professor of Pediatrics and Pharmacology, University of Pennsylvania School of Medicine Specific Aims 1. Triglycidyl Amine (TGA)—A new crosslinking reagent for preparing heart valve bioprostheses: Chemical and biological mechanisms 2. TGA-Matricellular interactions: Cellular and molecular biology studies related to anticalcification mechanisms. 3. Mechanisms responsible for TGA-mediated inhibition of heart valve calcification: Biomechanics, biocompatibility, & changes in gene expression patterns. Subcontract PI: Joseph Gorman, M. D. , Asst. Prof. Surg. Univ. Penn Sch. Med. Subcontract PI: Michael Sacks, Ph. D. , Prof. of Bioengineering, Univ. Pitt. The Joseph Stokes, Jr. , Research Institute
![Bioprosthetic Heart Valves Carpentier. Hancock Ionescu-Shiley Edwards [Medtronic] Porcine aortic valve Bovine pericardium The Bioprosthetic Heart Valves Carpentier. Hancock Ionescu-Shiley Edwards [Medtronic] Porcine aortic valve Bovine pericardium The](http://slidetodoc.com/presentation_image_h2/c7cf4f9e0ff1d89558f08c8752484dbc/image-6.jpg)
Bioprosthetic Heart Valves Carpentier. Hancock Ionescu-Shiley Edwards [Medtronic] Porcine aortic valve Bovine pericardium The Joseph Stokes, Jr. , Research Institute
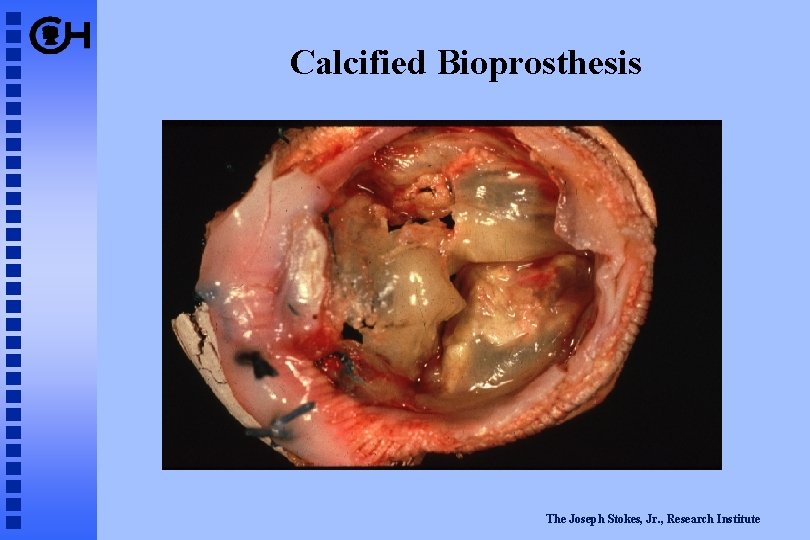
Calcified Bioprosthesis The Joseph Stokes, Jr. , Research Institute
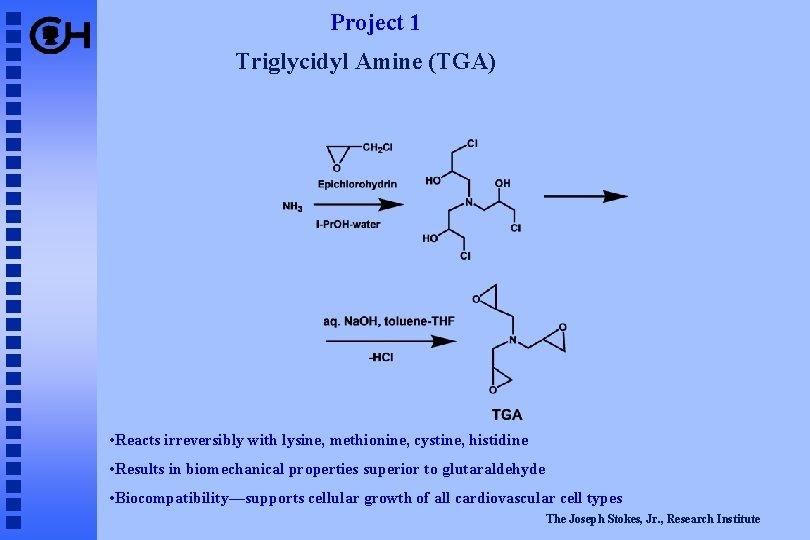
Project 1 Triglycidyl Amine (TGA) • Reacts irreversibly with lysine, methionine, cystine, histidine • Results in biomechanical properties superior to glutaraldehyde • Biocompatibility—supports cellular growth of all cardiovascular cell types The Joseph Stokes, Jr. , Research Institute
Project 2: Genetic Analysis of Human Outflow Tract Malformations Project Leader: Deborah Driscoll, M. D. , Professor and Chair, Department of Obstetrics and Gynecology, University of Pennsylvania School of Medicine Specific Aims 1. Developing a map of single nucleotide polymorphisms (SNP’s) in selected genes implicated in cardiac development. 2. Evaluating SNP’s for potential functional alterations. 3. Determining the genetic contribution of selected genes to the development of outflow tract malformations using family-based linkage disequilibrium testing. 4. Identifying modifiers of the cardiac phenotype in patients with 22 q 11 deletions The Joseph Stokes, Jr. , Research Institute
VEGF Related Directions Animal models demonstrate VEGF capable of influencing pharyngeal arch patterning SNPs with reduced VEGF expression are associated with cardiovascular defects in 22 q 11 deletion syndrome Identified an “at risk” haplotype for cardiovascular defects among individuals with 22 q 11 deletion Suggests risk of CHD in the fetus with 22 q 11 deletion increases when VEGF levels fall below critical threshold needed for proper development of pharyngeal arch arteries, severity may be due to degree of vascular impairment The Joseph Stokes, Jr. , Research Institute

Project 3: Genotype and Clinical Outcome in Conotruncal Defects Project Leader: Elizabeth Goldmuntz, M. D. , Associate Professor of Pediatrics, University of Pennsylvania School of Medicine Specific Aims 1. Investigations of the contribution of NKX 2. 5 and related genes to the etiology of controtruncal defects using mutation analyses and familybased association studies. 2. Investigating whether subsets of patients with transposition of the great arteries or double outlet right ventricle share a common genetic etiology with the heterotaxy syndrome: Studies of CFC 1 mutations & other genes (NODAL, ZIC 3, LEFTY 1, ACVRIIB) 3. Studies of the relationship between genetic etiology and clinical variability/outcome in subjects with conotruncal defects The Joseph Stokes, Jr. , Research Institute
Project 3: Aim 3 Impact of Genotype on Clinical Status Cross sectional study Subjects with TOF, Truncus or IAA Ages 8 -18 yo Clinical Assessment Exercise study Echocardiogram Cardiac MRI Child health questionnaire The Joseph Stokes, Jr. , Research Institute
Project 4: Molecular Analysis of Human Subtelomeric Rearrangements Project Leader: Ian Krantz, M. D. , Assistant Professor of Pediatrics and Genetics, University of Pennsylvania School of Medicine Specific Aims 1. Identify individuals with subtelomeric chromosomal deletions and congenital heart defects. 2. Develop a diagnostic assay that targets the critical region and sizes subsequent rearrangements of each of the telomeres. 3. Defining the critical regions and identifying candidate disease related genes for specific clinical phenotypes by mapping the extent and composition of the associated rearrangements. The Joseph Stokes, Jr. , Research Institute
Project 5: Chromosomal Rearrangements (CR) and Cardiac Candidate Genes Project Leader: Beverly S. Emanuel, Ph. D. , Professor and Chair of Genetics (at CHOP), University of Pennsylvania School of Medicine Specific Aims 1. Identify and characterize CR’s in patients with congenital heart disease by high-resolution cytogenetics and molecular cytogenetic analysis 2. Develop PCR-based mapping strategies using the human genomic sequence to identify the translocation BP’s 3. Characterize the genomic DNA from normal chromosomes at the chromosomal breakpoints in order to identify mechanisms of rearrangement 4. Identify the candidate genes disrupted or deleted at the translocation BP’s as candidates for early cardiac morphogenesis. 5. Determine whether mutations in the candidate genes are associated with the specific cardiac defect in other patients in the SCCOR Clinical Core. The Joseph Stokes, Jr. , Research Institute

The Clinical Core (a continuing resource from the SCOR’s) Director: Elizabeth Goldmuntz, M. D. Objectives: 1. Molecular analyses of the genetic etiology of conotruncal defects 2. Molecular analyses of bioprostheses 3. Impact of genotype on cardiac anatomy and clinical outcome Services 1. Ascertain Subjects 2. Acquisition of clinical data 3. Acquisition of relevant samples 4. Review of pertinent medical records 5. Coordination of clinical studies The Joseph Stokes, Jr. , Research Institute

The Cell Culture, DNA, and Microarray Core (a continuing resource from the SCOR’s) Director: Beverly Emanuel, Ph. D. Objectives: 1. Provide cell culture, DNA isolation, cytogenetic and DNA analysis support for all of the projects 2. Provide microarray resources 3. Training and consultation services to all Projects and Cores Services: 1. Establishing lympholastoid cell lines from patients with congenital heart defects. 2. Isolation of DNA from established cell lines, peripheral lymphocytes 3. Perform FISH to screen for 22 q 11. 2 deletions 4. Regionally localize newly identified human c. DNAs by FISH 5. Provide genotyping services for the SCCOR The Joseph Stokes, Jr. , Research Institute
Cardiac Morphology, Gene Expression and Histology Core Director: Kenneth Ryan, Ph. D. , Assistant Professor of Pediatrics, University of Pennsylvania School of Medicine Objectives: 1. Analysis of gene (m. RNA and protein) expression 2. Histological support 3. Breed mice & xenopus for harvesting embryos for whole-mount in situ hybridizations and sectioning re. cardiac gene expression patterns Services 1. Generate and bank frozen staged mouse & xenopus embryo RNA samples. 2. Generate as blocks and slides embedded mouse and xenopus embryos. 3. Share expertise in and perform in situ hybridizations using antisense RNA probes. 4. Perform immunohistochemistry and related histology The Joseph Stokes, Jr. , Research Institute

Bioinformatics and Data Analysis Core Director: Peter White, Ph. D. , Assistant Prof. Ped. , Univ. Penn. School of Medicine, Director, CHOP’s Bioinformatics Core Co-Director: Charles Scott, Ph. D. Objectives: 1. State of the art bioinformatics & biostatistics resources 2. Experimental design support 3. Data base development and data management support 4. The integration of bioinformatics and biostatistics data on SCCOR subjects Services 1. Provide assistance in study design, database design, and data storage 2. To provide infrastructure, hardware, software, technical support, for analyzing results of molecular biology experiments 3. Statistical analysis and data interpretation. The Joseph Stokes, Jr. , Research Institute

NHLBI PEDIATRIC HEART DISEASE SCCOR’s: 2005 Programmatic Meeting At the NHLBI, December 13, 2005 Children’s Hospital, Boston PI: Jane Newburger, M. D. Children’s Hospital of Cincinnati PI: Woody Benson, M. D. , Ph. D. Children’s Hospital of Pittsburgh Steve Webber, M. D. , Ph. D. The Joseph Stokes, Jr. , Research Institute
From Molecular Mechanisms to Improved Outcomes in TOF Jane W. Newburger, M. D. , M. P. H. Children’s Hospital, Boston Harvard Medical School The Joseph Stokes, Jr. , Research Institute
Children’s Hospital, Boston: Projects Project 1: Neurologic and developmental outcome in TOF (Newburger) Project 2: Randomized trial of pulmonary valve replacement in TOF (Geva) Project 3: Human mutations that cause TOF (Seidman) Project 4: Mitochondria in hypertrophied RV and surgical ischemia (Mc. Gowan) Project 5: Functional analysis of cardiac transcription factor NKX 2. 5 (Izumo/Jay) Project 6: Cardiac regeneration in zebrafish (Keating) The Joseph Stokes, Jr. , Research Institute
Cores Core A: Core B: Research support and statistics Microarray core (Newburger) (Schinke) Core C: Children’s Hospital-Harvard TOF registry (Breitbart) Core D: Skills development core: The pathology of CHD (Collins/Jurazek) The Joseph Stokes, Jr. , Research Institute
Children’s Hospital of Cincinnati: SCCOR in Pediatric Heart Disease: PI Woody Benson “Molecular mechanisms of valve development and disease” PI: D. Woodrow Benson, MD, Ph. D Project 1 – Benson—Genetic Studies of Valvular Heart Disease Project 2 – Gelb—To Identify PTPN 11 Defects Associated with Noonan’s syndrome and other forms of congenital heart disease. Project 3 – Yutzey--Regulation of valvuloseptal development by DSCR 1 Project 4 – Robbins-- Mechanisms of Cardiac pathogenesis in Noonan Syndrome, effects of SHP-2 Gln 79 Arg, a common PTPN 11 mutation associated with Noonan’s syndrome. The Joseph Stokes, Jr. , Research Institute
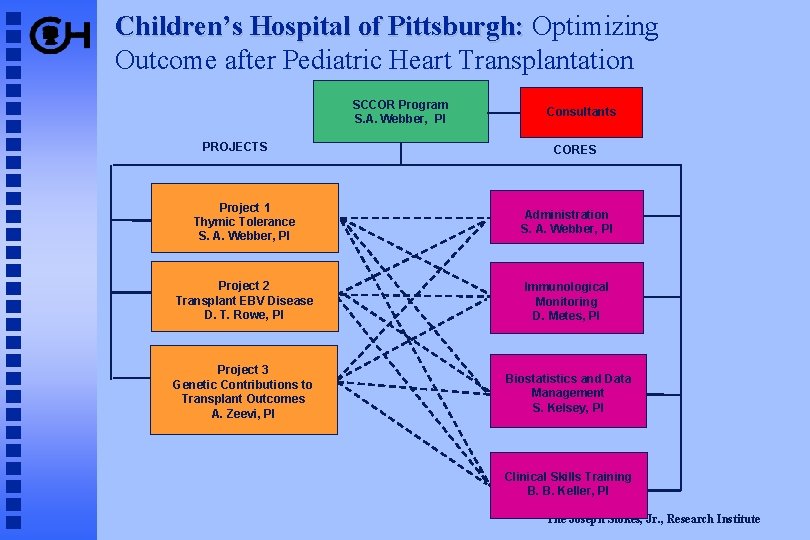
Children’s Hospital of Pittsburgh: Optimizing Outcome after Pediatric Heart Transplantation SCCOR Program S. A. Webber, PI PROJECTS Consultants CORES Project 1 Thymic Tolerance S. A. Webber, PI Administration S. A. Webber, PI Project 2 Transplant EBV Disease D. T. Rowe, PI Immunological Monitoring D. Metes, PI Project 3 Genetic Contributions to Transplant Outcomes A. Zeevi, PI Biostatistics and Data Management S. Kelsey, PI Clinical Skills Training B. B. Keller, PI The Joseph Stokes, Jr. , Research Institute
- Slides: 24