The New Drug RD Technical Guidelines Development in











- Slides: 15

The New Drug R&D Technical Guidelines Development in China – Perception of RDPAC Member Companies May 17, 2011 Beijing, China Maggie Chang Director – Drug Regulatory & Medical Affairs China Association of Enterprises with Foreign Investment R&D-based Pharmaceutical Association Committee (RDPAC)

Outline n n RDPAC introduction ICH technical guidelines n n n Other technical guidelines n n Implementation in China Introduction of China ICH Study Group Technical Guidelines Construction in China Translation of foreign technical guidelines Recommendation of technical guidelines development in China Closing remarks Drug Information Association 2

Healthier China Through Innovation 创新引领健康中国 Healthier China Through Innovation 为提高患者的生活质量提供医疗保健解决方案 以创新的医疗保健产品和投资,为中国的高速增长提供支持 Providing healthcare solutions to improve patients' quality of life Contributing to the rapid growth of China through innovative healthcare offers and investment

37 Members are R&D-based Pharmaceutical Companies US (13) Abbott Baxter Biogen Idec Bristol Myers Squibb Cephalon Celgene Eli Lilly GE Healthcare Genzyme MSD Pfizer UCB Xian-Janssen EU (18) Astra. Zeneca Bayer Health. Care Boehringer Ingelheim Fresenius Kabi GSK Gedeon Richter Plc. Ipsen LEO Pharma Lundbeck Merck Serono Mundipharma Novartis Novo Nordisk Nycomed Roche Sanofi Aventis Servier UCB JP (6) Astellas Daiichi-Sankyo Eisai Santen Pharmaceutical Sumitomo Takeda AU (1) CSL Biotherapies

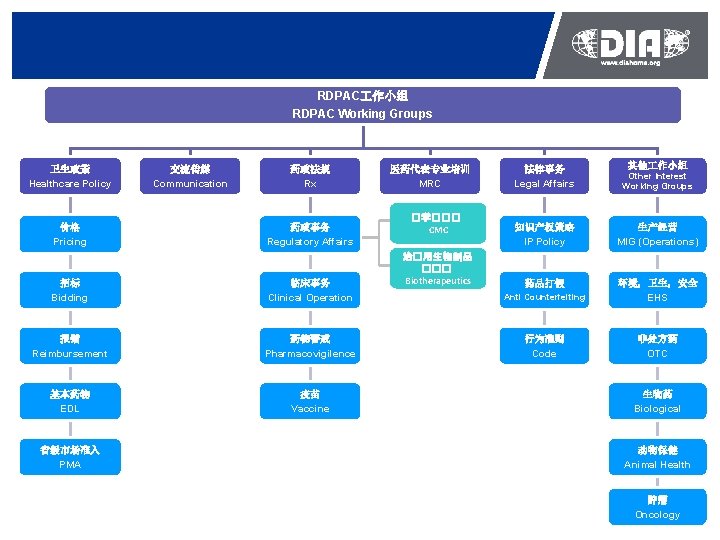
RDPAC 作小组 RDPAC Working Groups 卫生政策 Healthcare Policy 价格 Pricing 交流传媒 Communication 药政法规 Rx 药政事务 Regulatory Affairs 招标 Bidding 临床事务 Clinical Operation 报销 Reimbursement 药物警戒 Pharmacovigilence 基本药物 EDL 疫苗 Vaccine 省级市场准入 PMA 医药代表专业培训 MRC �学��� CMC 治�用生物制品 ��� Biotherapeutics 其他 作小组 法律事务 Legal Affairs Other Interest Working Groups 知识产权策略 IP Policy 生产经营 MIG (Operations) 药品打假 Anti Counterfeiting 环境,卫生,安全 EHS 行为准则 Code 非处方药 OTC 生物药 Biological 动物保健 Animal Health 肿瘤 Oncology

ICH Technical Guidelines Implementation in China n n ICH technical guidelines are well considered during review Applicants are encouraged to submit CTD format ICH technical guidelines development are closely followed Efforts of SFDA/CDE are highly appreciated by RDPAC Member companies Drug Information Association 6

Introduction of China ICH Study Group n Established in 2009, aimed to n n n Study the ICH technical guidelines in a systematically manner , considering the situation in China, to transform to the technical guidelines suitable for China, organize training and promulgation activities Follow the progress and changes of ICH technical guidelines, enhance the exchange and cooperation with International Organizations, provide comments and suggestions from the perceptive of China Boost the continuous improvement of the administration of drug R&D and registration Led by SFDA, participated by SFDA Technical Affiliates, including NIFDC, CPC, CDE, CCD, CDR, CQAP and RDPAC and academia, experts from local leading R&D-based drug manufacturers to be invited Major areas including Quality, Safety, Efficacy and Multidisciplinary CCD: Center for Drug Certification CDR: Center for Drug Reevaluation CQAP: China Quality Association for Pharmaceuticals NIFDC: National Institute for Food and Drug Control CDE: Center for Drug Evaluation CPC: Chinese Pharmacopoeia Commission RDPAC: R&D based Pharmaceutical Association Committee

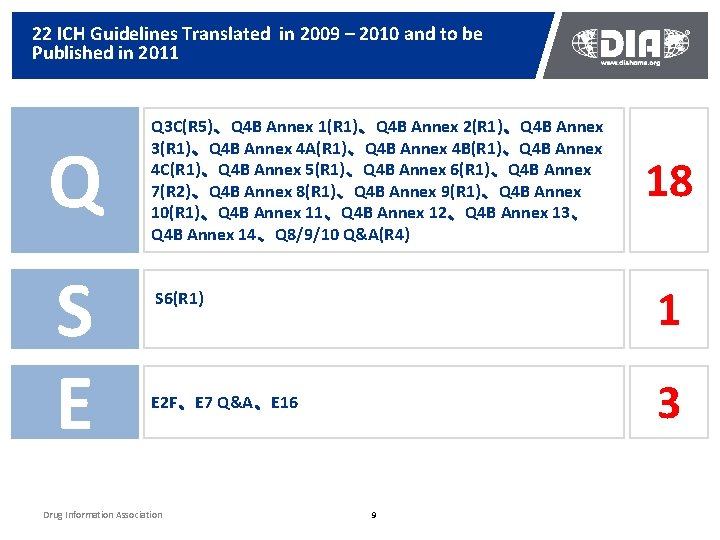
Major Progress of China ICH Study Group n n n Working procedures formulated, priorities identified Studies and training ongoing as planned, webpage and Annual Report 2010 under development Regular meetings held twice a year Establishment and activities reported ICH Steering Committee and ICH GCG in 2010 22 ICH guidelines translated and to be published in 2011

22 ICH Guidelines Translated in 2009 – 2010 and to be Published in 2011 Q S E Q 3 C(R 5)、Q 4 B Annex 1(R 1)、Q 4 B Annex 2(R 1)、Q 4 B Annex 3(R 1)、Q 4 B Annex 4 A(R 1)、Q 4 B Annex 4 B(R 1)、Q 4 B Annex 4 C(R 1)、Q 4 B Annex 5(R 1)、Q 4 B Annex 6(R 1)、Q 4 B Annex 7(R 2)、Q 4 B Annex 8(R 1)、Q 4 B Annex 9(R 1)、Q 4 B Annex 10(R 1)、Q 4 B Annex 11、Q 4 B Annex 12、Q 4 B Annex 13、 Q 4 B Annex 14、Q 8/9/10 Q&A(R 4) 1 S 6(R 1) 3 E 2 F、E 7 Q&A、E 16 Drug Information Association 18 9

MSD’s Efforts on Translation and Publication of ICH Technical Guidelines (1/2) The 1 st Edition: The 2 nd Edition: The 3 rd Edition: Quality: 14 Quality: 23 (Guidance updated from 2006 to 2008) Efficacy: 14 Efficacy: 17 Safety: 13 Safety: 14 Quality: 6 Efficacy: 3 Safety: 4 2001 2007 2011 MSD’s initiative since 1999 and the continuous support and efforts are ongoing…… 1 common goal 10 + experts 100 + translators 108 technical guidelines translated Drug Information Association 10

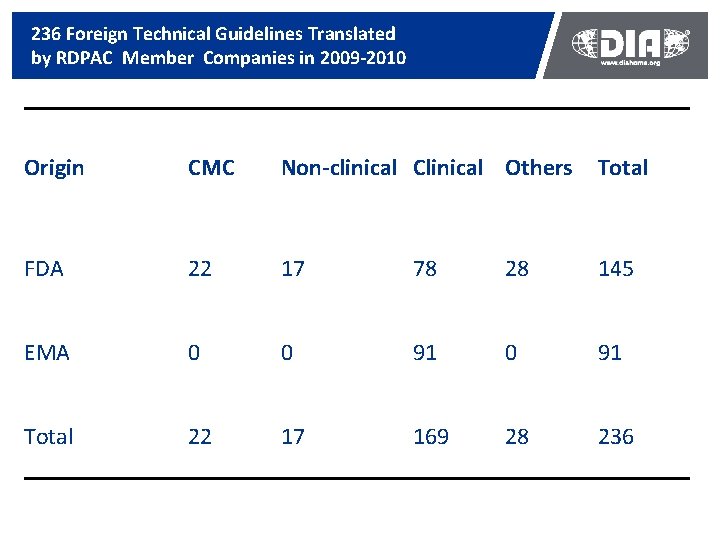
Technical Guidelines Construction Ongoing in China Encouraging Start n n n 75 technical guidelines officially published by SFDA 6 general guidelines officially published by CDE 13 technical standard officially published by CDE Drug Information Association Solid Progress n n n 11 236 foreign reference technical guidelines translated 147 foreign reference technical guidelines published by CDE 31 foreign technical guidelines under conversion

236 Foreign Technical Guidelines Translated by RDPAC Member Companies in 2009 -2010 Origin CMC Non-clinical Clinical Others Total FDA 22 17 78 28 145 EMA 0 0 91 Total 22 17 169 28 236

Recommendation of Technical Guidelines Development in China n n n Conversion of prioritized guidelines one by one and step by step Beginning to end involvement of experts from local companies and MNCs in a systemic way At least two round publication of draft for seeking public comments Development of compatible regulations In parallel translation of latest foreign technical guidelines Drug Information Association 13

Closing Remarks n RDPAC member companies are encouraged well by very positive movements of CDE recently n n n positive re-structuring of the CDE “Principles and Procedures for Drug Review” published by – a milestone CTD Format submission China ICH Study Group is play an important role to introduce ICH technical guidelines into practice in China Technical guidelines construction in China is a long term project, important for all R&D based pharmaceutical companies in China RDPAC Member Companies are prepared to support SFDA/CDE for the development of technical guidelines in China Drug Information Association 14

Many thanks for your kind attention! maggiechang@rdpac. org www. rdpac. org