The Nature of Science Science is the use

















































- Slides: 67

The Nature of Science

Science is the use of evidence to construct testable explanations and predictions of natural phenomena, as well as the knowledge generated through this process. Science only answers questions that are testable by a process called scientific inquiry- the planned and deliberate investigation of the natural world

LABORATORY INVESTIGATIONS There a variety of ways to conduct a laboratory investigation depending on the desired outcome. Comparative A comparison of 2 or more things Descriptive Observational lab; includes two types of data Quantitative: involve numbers, measurements, quantities Qualitative: descriptions information is obtained Experimental Designed experiment that follows the scientific method Clearly defined control and test group

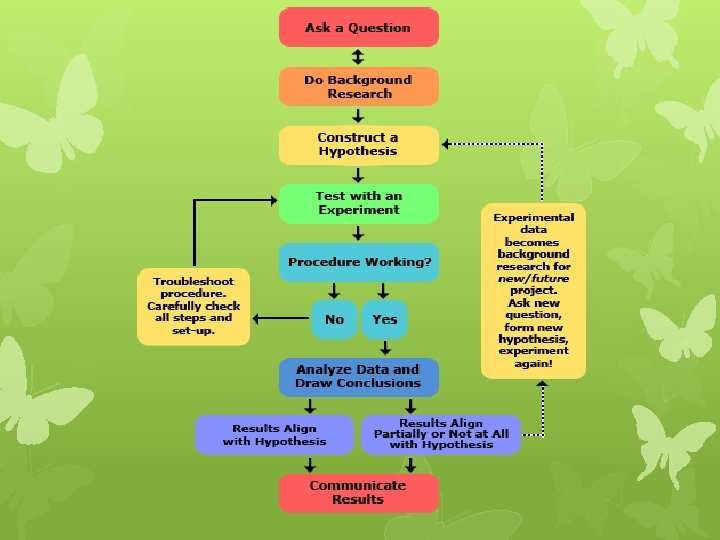
THE SCIENTIFIC METHOD misleading because it is process not reserved for biologist and other scientists also is it not a methodical set of steps to be followed in a specific order it is an organized pattern of thinking to solve everyday problems it is problem-solving technique that involves:


Which is NOT an example of a hypothesis? A. If I raise the temperature of a cup of water, then the amount of sugar that can be dissolved in it will be increased B. Ladybugs are a good natural pesticide for treating aphid infected plants. C. When there is less oxygen in the water, rainbow trout suffer more lice. D. If temperature is related to the rate of metabolism in animals, then raising the ambient temperature will cause an increase in animal metabolism.

The Experiment Control Group: is a setup used for comparison Experimental Group: the group exposed to the factor being tested Variable- factor changed by the experimenter. Only one factor should be changed at a time. Independent (Manipulated) Variable-The factor used to test the hypothesis; it might affect the outcome of the experiment. Dependent (Responding )Variable: it results from or depends on changes to the independent variable. Constant: Factors that remains fixed during an experiment while the independent and dependent variables changes.

Hypothesis: Puppies that are given vitamins gain more weight. Half the puppies aren’t given vitamins. Half the Puppies are given vitamins. Which of the following would be the dependent variable? A. Vitamins B. Weight gain C. Puppies D. No Vitamins

Hypothesis: Puppies that are given vitamins gain more weight. Half the puppies aren’t given vitamins. Half the Puppies are given vitamins. Which of the following would be the Control? A. Vitamins B. Weight gain C. Puppies D. No Vitamins

Hypothesis: Puppies that are given vitamins gain more weight. Half the puppies aren’t given vitamins. Half the Puppies are given vitamins. Which of the following would be the Independent variable? A. Vitamins B. Weight gain C. Puppies D. No Vitamins

Collecting Data- Information gained from observations Qualitative- data that are descriptions of what our senses detect You collect by observing, looking & listening. Ex: Colors, textures, smells, tastes, appearance, beauty Quantitative- data collected as numbers Collected by measuring Length, height, area, volume, weight, speed, time, temperature, humidity, sound levels, cost, members, ages, etc

What type of Data is this? Freshman Class friendly demeanors civic minded environmentalists positive school spirit A. Quantitative data B. Qualitative data

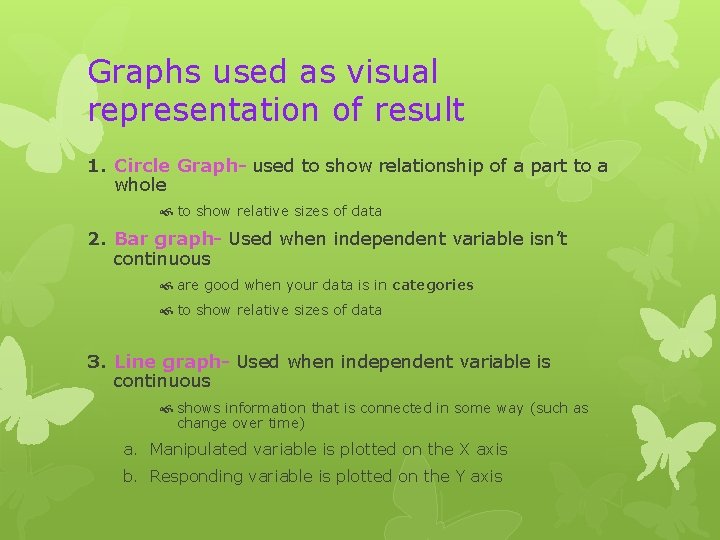
Graphs used as visual representation of result 1. Circle Graph- used to show relationship of a part to a whole to show relative sizes of data 2. Bar graph- Used when independent variable isn’t continuous are good when your data is in categories to show relative sizes of data 3. Line graph- Used when independent variable is continuous shows information that is connected in some way (such as change over time) a. Manipulated variable is plotted on the X axis b. Responding variable is plotted on the Y axis

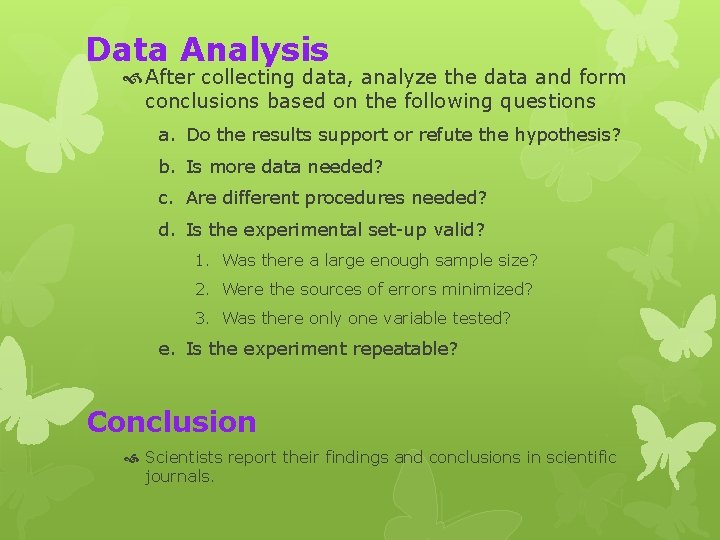
Data Analysis After collecting data, analyze the data and form conclusions based on the following questions a. Do the results support or refute the hypothesis? b. Is more data needed? c. Are different procedures needed? d. Is the experimental set-up valid? 1. Was there a large enough sample size? 2. Were the sources of errors minimized? 3. Was there only one variable tested? e. Is the experiment repeatable? Conclusion Scientists report their findings and conclusions in scientific journals.

Scientific Theory An explanation of natural or physical phenomenon supported by many observations and experiments over time. Tested by multiple independent researchers. Complex explanations and have been supported by years of scientific research They are considered valid until new areas of study are developed or until new technologies are developed and new evidence is found.

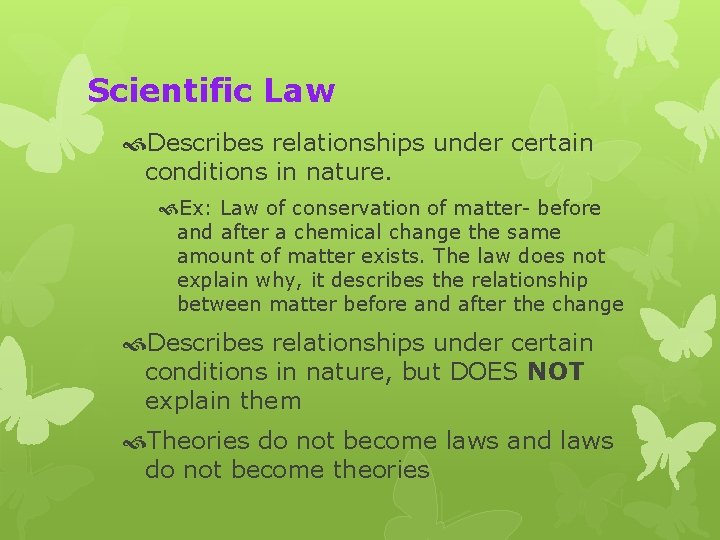
Scientific Law Describes relationships under certain conditions in nature. Ex: Law of conservation of matter- before and after a chemical change the same amount of matter exists. The law does not explain why, it describes the relationship between matter before and after the change Describes relationships under certain conditions in nature, but DOES NOT explain them Theories do not become laws and laws do not become theories

Scientific Theory vs. Hypothesis When a hypothesis is tested many times and the explanation is durable, the hypothesis can be incorporated into a theory. A Scientific Theory is a well-established and highly reliable explanation

INTRODUCTION TO BIOLOGY

INTRODUCTION TO BIOLOGY A. What is Biology? Biology means the study of life. Biology is the science that seeks to understand the living world. Bio = Life ology =Study of

Characteristics of Life 1. Living things are made of Cells. a. A cell is the smallest working unit of life. b. Living organisms are grouped by the number of cells: * Unicellular – single-celled organisms; ex. Bacteria, amoebas * Multicellular – organisms made up of more than one cell; ex. Humans, frogs, fish, insects, plants

Characteristics of Life Cells Tissue Organ system Organism 2. Living things Displays Organization a. A cell is a collection of organized structures that carries on life functions b. All living structures are composed of atoms and molecules. c. In multicellular organisms i. Specialized cells are organized into groups that work together called tissues. ii. Tissues are organized into organs, iii. Organ systems work together to support an organism

Characteristics of Life 3. Living things Grow & Develop a. Growth- all living things grow at least part of their lives, single-celled organism simple increase in size b. Development- describes physical changes that take place during the lifetime of an organism

Characteristics of Life 4. Living things Reproduce a. Not essential for individual organisms, but essential for the species b. Species- group of organisms that can breed with one another and produce fertile offspring c. Two ways: i. Asexual- new organism has a single parent; example - single-celled organism splits in half NO EXCHANGE OF GENETIC MATERIAL ii. Sexual- two cells ( egg & sperm) from different parents unit to form an embryo There IS AN EXCHANGE OF GENETIC MATERIAL

Characteristics of Life 5. Living things Respond to Stimuli a. External stimulus- includes all things that are OUTSIDE the organism. Ex: temperature, light b. Internal stimulus- all things that are INSIDE the organism. Ex: hunger, thirst

Characteristics of Life 6. Living things Require Energy a. two main ways to obtain energy i. Photosynthesis- energy from the sun. Plants, some bacteria & protist use this process ii. Consumer- energy from the food they eat. Us, other animals & fungi b. All organisms use energy for metabolismchemical reactions which builds up or breaks down materials as it carries out its life processes.

Characteristics of Life 7. Living things Maintain Homeostasis a. process by which organisms maintain a relatively stable internal environment

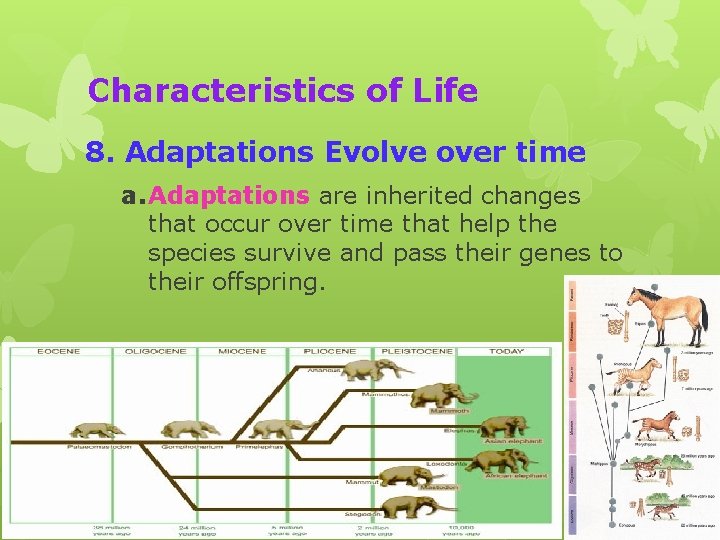
Characteristics of Life 8. Adaptations Evolve over time a. Adaptations are inherited changes that occur over time that help the species survive and pass their genes to their offspring.

REMEMBER: Cells Homeostasis Adaptation/evolution Reproduction Metabolism I-stimuli N-Organization Growth & Development

Which of these best describes an organism’s ability to maintain the constant internal conditions necessary for life? A. Homeostasis [Default] [MC Any] [MC All] B. Stability C. Reproduction D. Adaptation

Which two characteristics of life are important to the species but NOT to the individual A. Evolution & Growth and development B. Evolution & Reproduction C. Reproduction & Respond to the environment [Default] [MC Any] [MC All] D. Respond to the environment & Growth and development

chemical reactions which builds up or breaks down materials A. Metabolism B. Homeostasis C. Reproduction D. Response to stimuli [Default] [MC Any] [MC All]

When a ball is thrown at you and you move, this is an example of which characteristic? A. Homeostasis B. Reproduction C. Adaptation D. Response to stimuli [Default] [MC Any] [MC All]

For years people have been using pesticides to kill lice. Well some lice are able to survive the pesticides and are able to reproduce. Their offspring are also immune to the pesticide. This is an example of? A. Growth and development [Default] [MC Any] [MC All] B. Adapt/Evolve C. Reproduction D. Homeostasis

A caterpillar changes into a butterfly A. Reproduction B. Adaptation C. Growth and development D. Organization [Default] [MC Any] [MC All]

Biochemistry

Hierarchy of Life 1. Atom - Smallest unit of matter that retains its elemental properties 2. Molecule - Groups of atoms bonded together 3. Cell - Smallest working unit of life 4. Organism - Individual living thing; depending on the complexity, an organism may be composed of a. Tissue - groups of cells working together b. Organ – groups of tissues working together c. Organ system - groups of organs working together 5. Population - Group of organisms of one species in one area 6. Community - Different populations that live together in a specific area 7. Ecosystem - A community and its non-living components 8. Biosphere – Earth

What is the smallest working unit of Life? A. Atom B. Cell C. Tissue [Default] [MC Any] [MC All] D. Organ

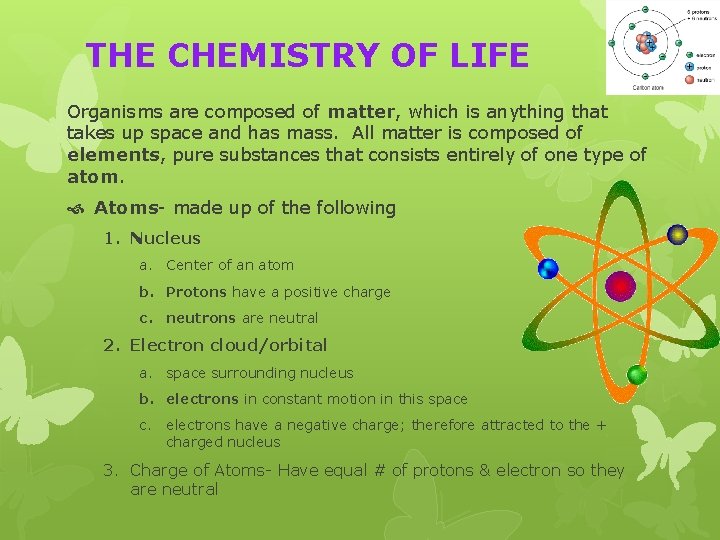
THE CHEMISTRY OF LIFE Organisms are composed of matter, which is anything that takes up space and has mass. All matter is composed of elements, pure substances that consists entirely of one type of atom. Atoms- made up of the following 1. Nucleus a. Center of an atom b. Protons have a positive charge c. neutrons are neutral 2. Electron cloud/orbital a. space surrounding nucleus b. electrons in constant motion in this space c. electrons have a negative charge; therefore attracted to the + charged nucleus 3. Charge of Atoms- Have equal # of protons & electron so they are neutral

Elements 1. 92 naturally occurring elements 2. 25 essential to life 3. 4 making up 96% of living matter: carbon, hydrogen, oxygen, nitrogen 4. atomic # = # of protons that element contains

Compounds 1. elements combined in fixed ratios of atoms form compounds 2. held together by chemical bonds 3. Chemical formula a. shorthand to show elements in a compound 4. Chemical Equation a. recipe for making a compound b. Reactants- what goes into the reaction c. Products- substance that is formed

H 2 SO 4 How many H? How many S? How many O? A. 2 H, 2 S, 4 O B. 4 H, 1 S, 2 O C. 2 H, 1 S, 4 O D. 1 H, 2 S, 1 O

2 H 2 + O 2 2 H 2 O Reactants? Products? A. Reactant= 2 H 2 + O 2 Products= 2 H 2 O B. Reactant= 2 H 2 O Products= 2 H 2 + O 2

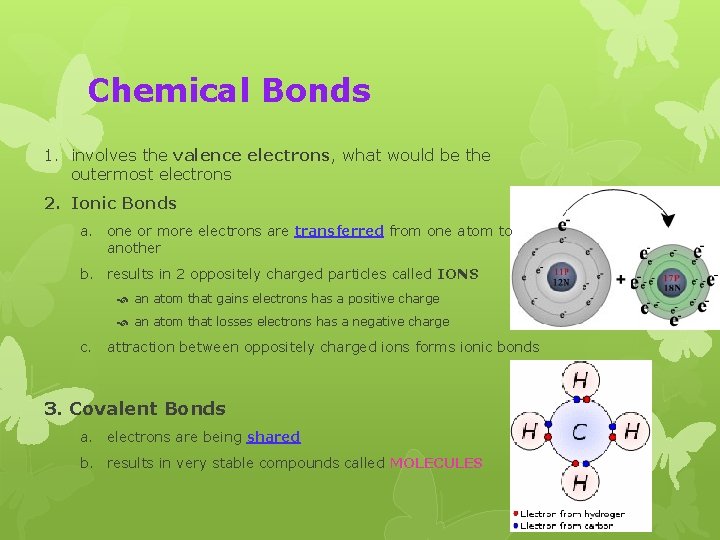
Chemical Bonds 1. involves the valence electrons, what would be the outermost electrons 2. Ionic Bonds a. one or more electrons are transferred from one atom to another b. results in 2 oppositely charged particles called IONS an atom that gains electrons has a positive charge an atom that losses electrons has a negative charge c. attraction between oppositely charged ions forms ionic bonds 3. Covalent Bonds a. electrons are being shared b. results in very stable compounds called MOLECULES


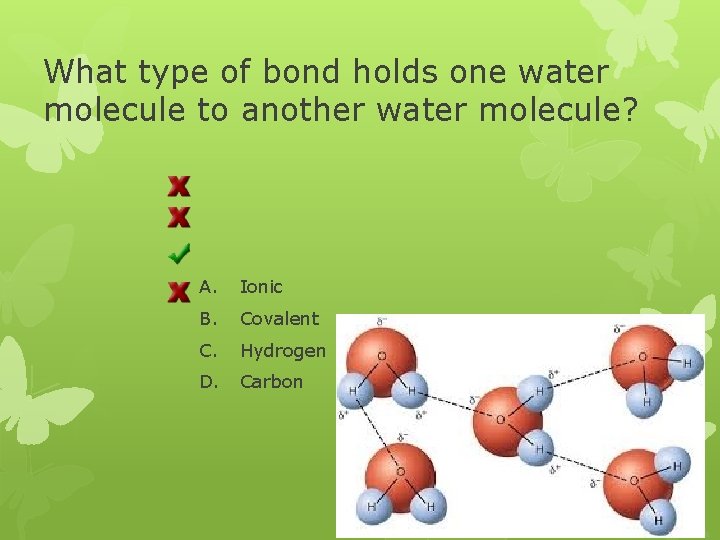
Water & Solutions Water is the most essential and abundant substance on Earth. Cells are made up of mostly water and most cells are surrounded by water. The importance of water is largely due to its unique characteristics, which all directly relate to one very important property of water. . . Water is polar.

What is the Equation for Water? A. H 2 O 2 B. OH C. HO 2 D. H 2 O

What does H 2 O Mean? A. There are 2 oxygens & 1 Hydrogen B. There are 2 Hydrogens & 1 Oxygen C. There is 1 Hydrogen & 1 Oxygen D. There are 2 Hydrogens & 2 Oxygens

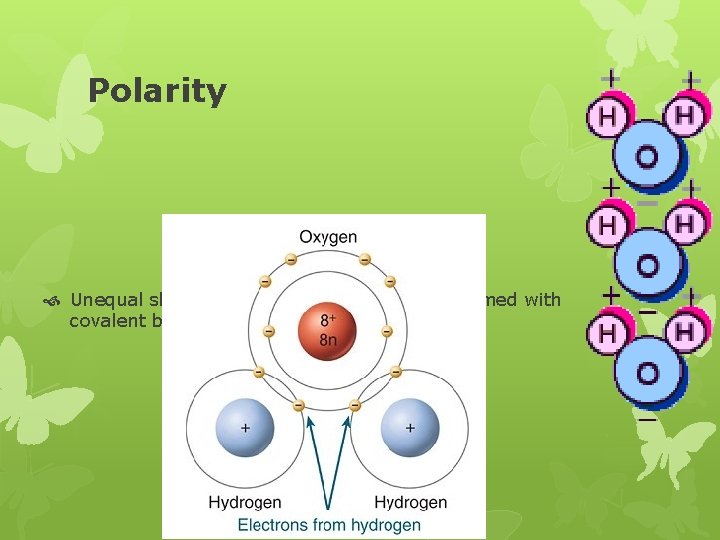
Polarity Unequal sharing of electrons in molecules formed with covalent bonds

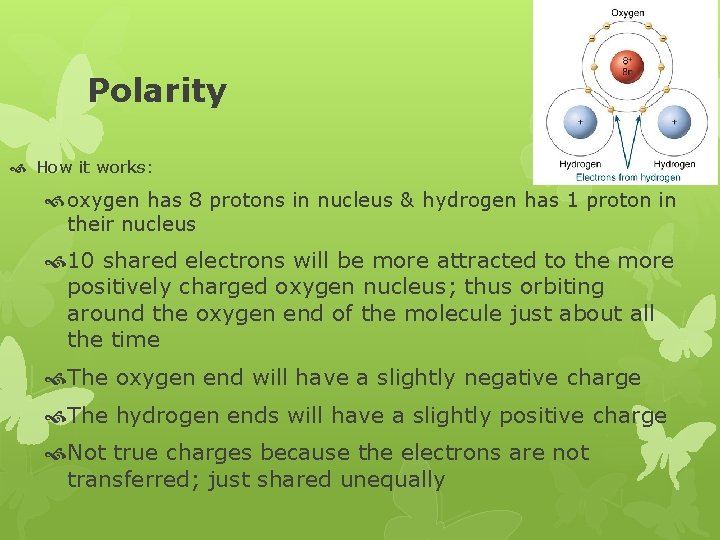
Polarity How it works: oxygen has 8 protons in nucleus & hydrogen has 1 proton in their nucleus 10 shared electrons will be more attracted to the more positively charged oxygen nucleus; thus orbiting around the oxygen end of the molecule just about all the time The oxygen end will have a slightly negative charge The hydrogen ends will have a slightly positive charge Not true charges because the electrons are not transferred; just shared unequally

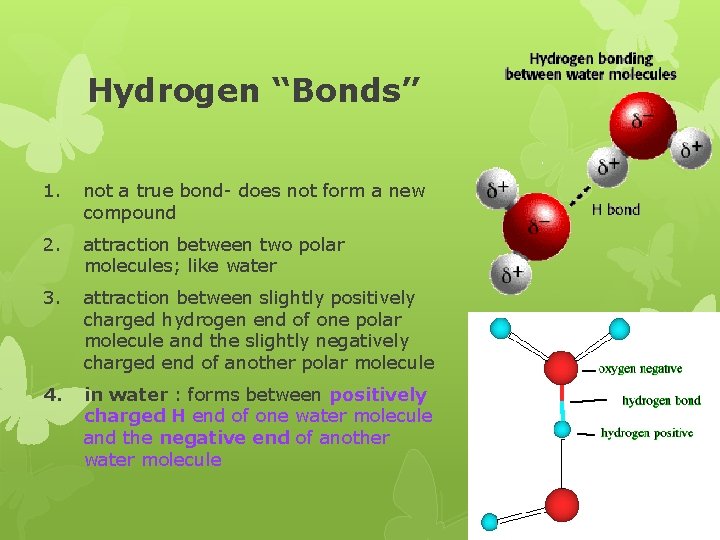
Hydrogen “Bonds” 1. not a true bond- does not form a new compound 2. attraction between two polar molecules; like water 3. attraction between slightly positively charged hydrogen end of one polar molecule and the slightly negatively charged end of another polar molecule 4. in water : forms between positively charged H end of one water molecule and the negative end of another water molecule

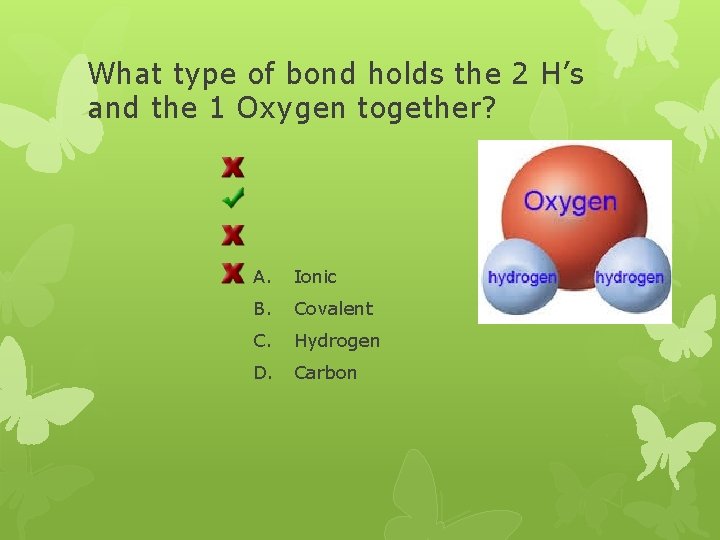
What type of bond holds the 2 H’s and the 1 Oxygen together? A. Ionic B. Covalent C. Hydrogen D. Carbon

What type of bond holds one water molecule to another water molecule? A. Ionic B. Covalent C. Hydrogen D. Carbon

What is Polarity? A. Unequal sharing of electrons B. When one atom has a slight positive charge and one has a slight negative charge C. Will form a hydrogen bond D. All of the above

Properties of Water 1. Cohesion or Surface tension a. Attraction between molecules of the same substance; water molecules stick together http: //www. reptilianagenda. com/img/pics/lizard. mov

Properties of Water 2. Adhesion or Capillary action a. attraction between molecules of different substances; water sticks to other surfaces

Properties of Water 3. Universal Solvent a. slightly charged ends of water attract and separate atoms of other compounds; dissolving them b. anything dissolved in water is called a solution c. many important substances in cells are in solution

Properties of Water 4. High heat of vaporization a. takes a lot of heat energy to evaporate a small amount of water

Properties of Water 5. High specific heat a. water absorbs heat energy without its temperature rising much

Properties of Water 6. Expansion on Freezing a. ice less dense than water; ice floats

Which property of water gives access to water at the top of trees? A. Cohesion B. Capillary action C. Universal Solvent D. Expansion on Freezing

In water, the O has a slight ____ charge A. Negative B. Positive C. Neutral D. Both A & B

Which property of water allows nutrients to be dissolved in blood? A. Capillary action B. Cohesion C. Universal Solvent D. Adhesion

What type of bond forms between two molecules of water? A. Ionic B. Covalent C. Hydrogen D. Wet

Which property to water allows animals to survive in the water during the HOT summer months without “cooking”? A. Cohesion B. High Heat of Vaporization C. High Specific Heat D. Expansion on Freezing

______ is the unequal sharing of electrons in water & causes it to have special properties A. Surface Tension B. High Heat of Vaporization C. Adhesion D. Polarity

Which property of water allows animals/fish to survive in the water during the COLD winter months? A. Expansion on Freezing B. High Specific Heat C. High Heat of Vaporization D. Universal Solvent

Which property of water allows some animals the ability to “walk” on water? A. Adhesion B. Cohesion C. Universal Solvent D. High Heat of Vaporization