The Nature of Radioactivity Rutherford 1871 1937 Nuclear










- Slides: 50

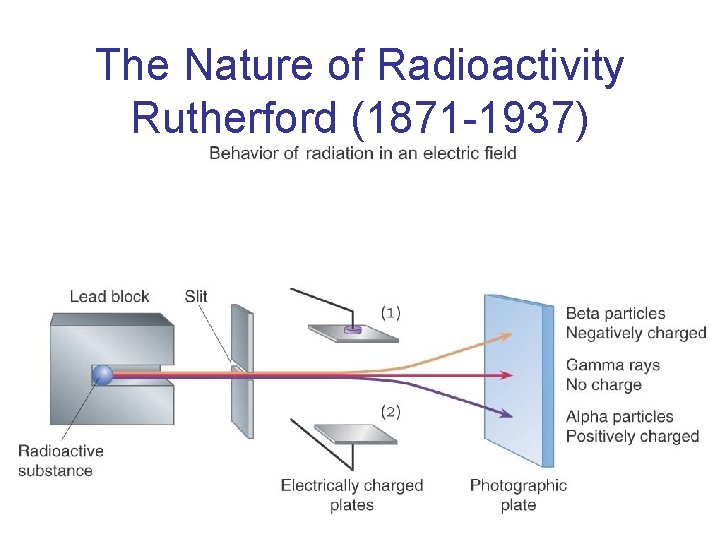
The Nature of Radioactivity Rutherford (1871 -1937)

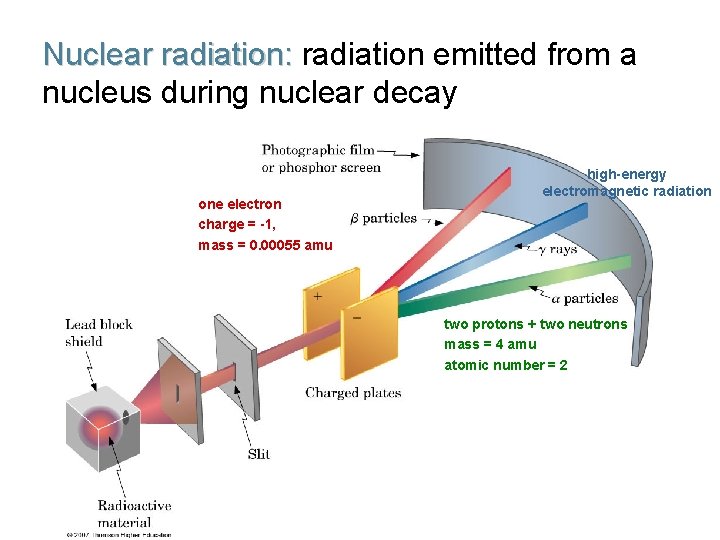
Nuclear radiation: radiation emitted from a nucleus during nuclear decay one electron charge = -1, mass = 0. 00055 amu high-energy electromagnetic radiation two protons + two neutrons mass = 4 amu atomic number = 2

Which kind of radiation is attracted toward a negatively charged plate? 1. 2. 3. 4. alpha particle beta particle gamma ray More than one answer is correct.

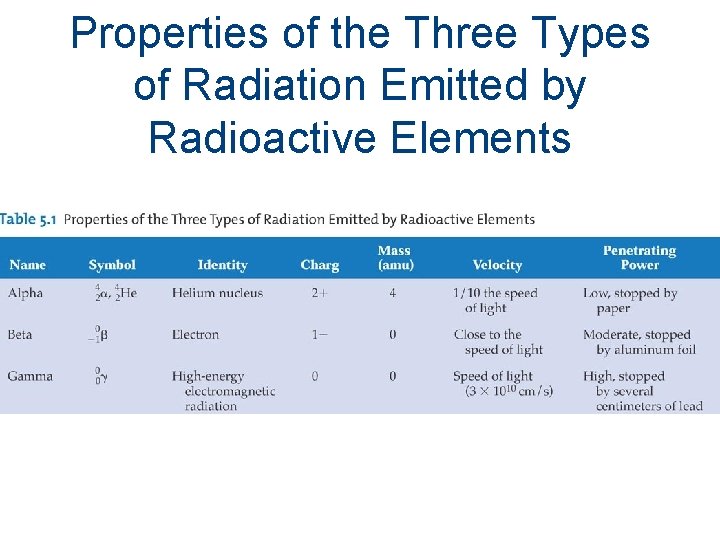
Properties of the Three Types of Radiation Emitted by Radioactive Elements

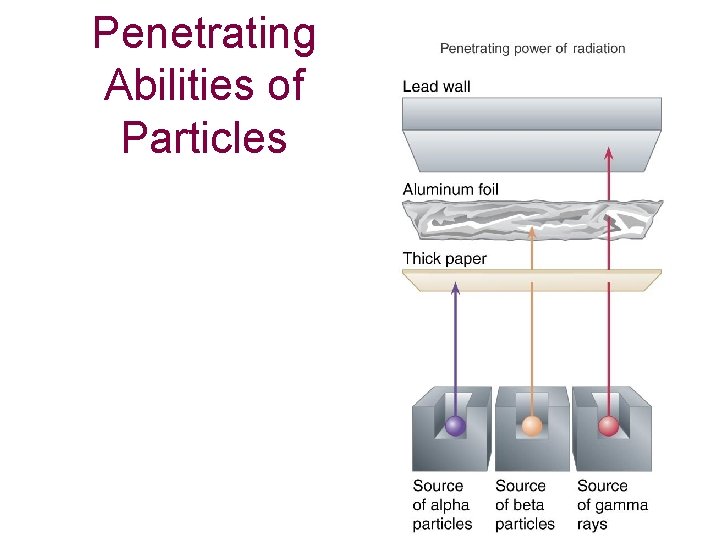
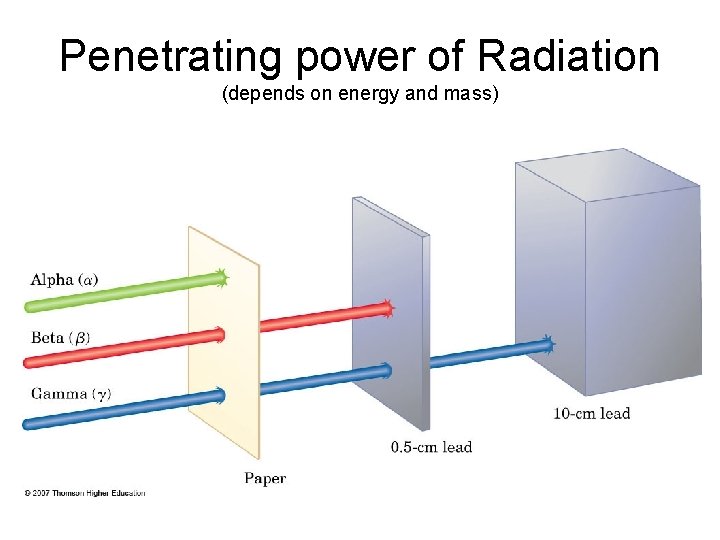
Penetrating Abilities of Particles

Penetrating power of Radiation (depends on energy and mass)

PENETRATING POWER a particles cause more damage than X-rays or g radiation but they have very low penetrating power and cannot pass through skin. Consequently alpha particles are not as harmful to humans or animals as long as they do not get into the body; if they do get into the body, they can be the most harmful. b particles are less damaging to tissue than a particles but penetrate farther and so are generally more harmful. g rays, which can easily penetrate skin, can be the most dangerous and harmful form of radiation.

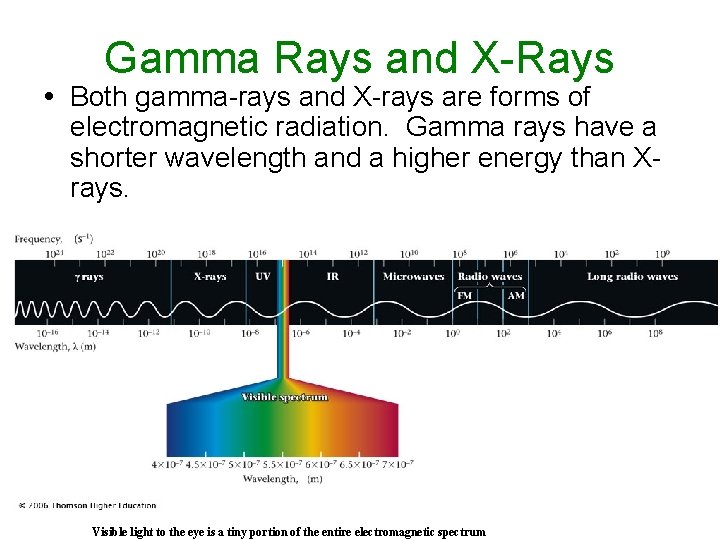
Gamma Rays and X-Rays Both gamma-rays and X-rays are forms of electromagnetic radiation. Gamma rays have a shorter wavelength and a higher energy than Xrays. Visible light to the eye is a tiny portion of the entire electromagnetic spectrum

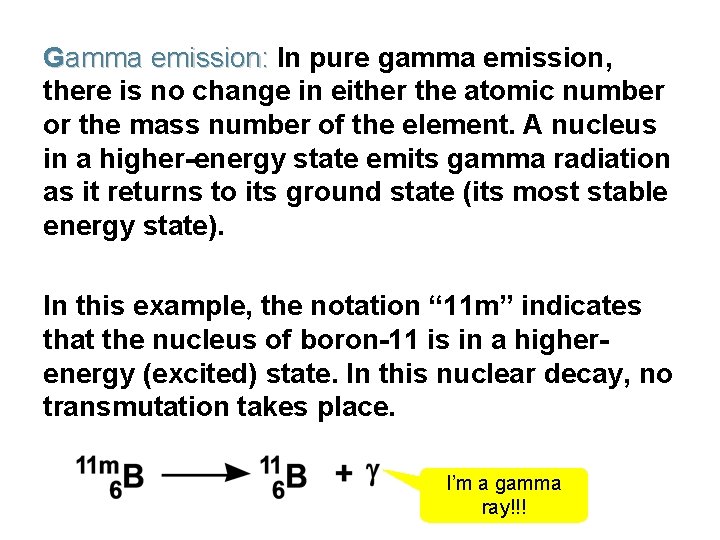
Gamma emission: In pure gamma emission, there is no change in either the atomic number or the mass number of the element. A nucleus in a higher-energy state emits gamma radiation as it returns to its ground state (its most stable energy state). In this example, the notation “ 11 m” indicates that the nucleus of boron-11 is in a higherenergy (excited) state. In this nuclear decay, no transmutation takes place. I’m a gamma ray!!!

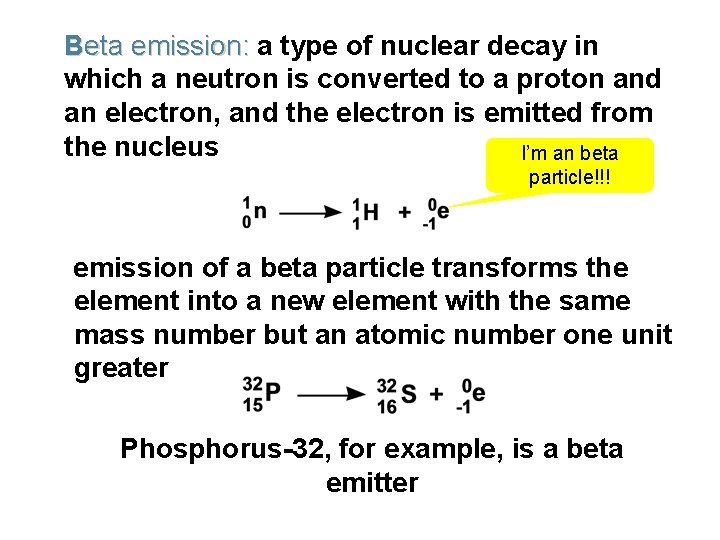
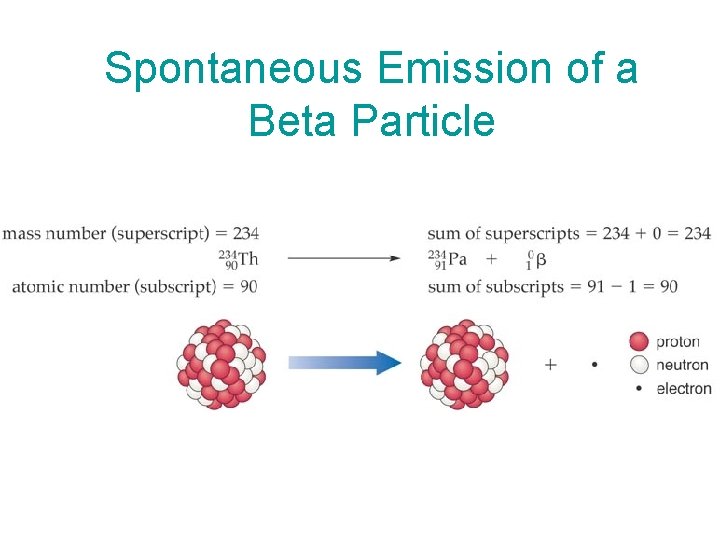
Beta emission: a type of nuclear decay in which a neutron is converted to a proton and an electron, and the electron is emitted from the nucleus I’m an beta particle!!! emission of a beta particle transforms the element into a new element with the same mass number but an atomic number one unit greater Phosphorus-32, for example, is a beta emitter

Spontaneous Emission of a Beta Particle

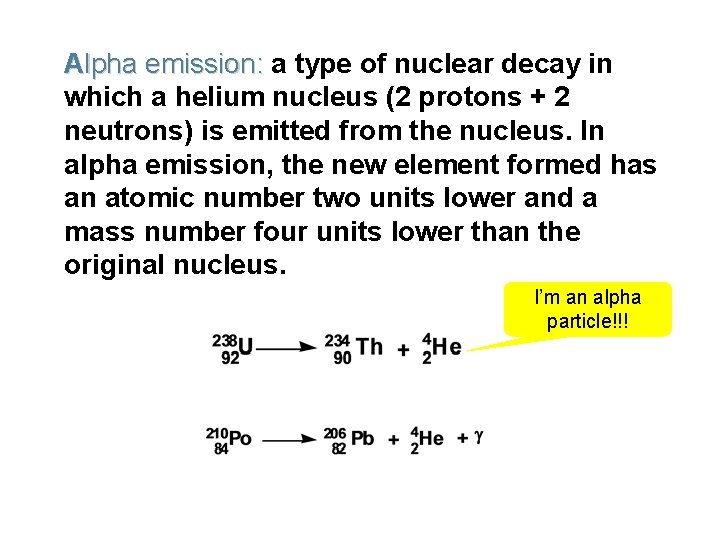
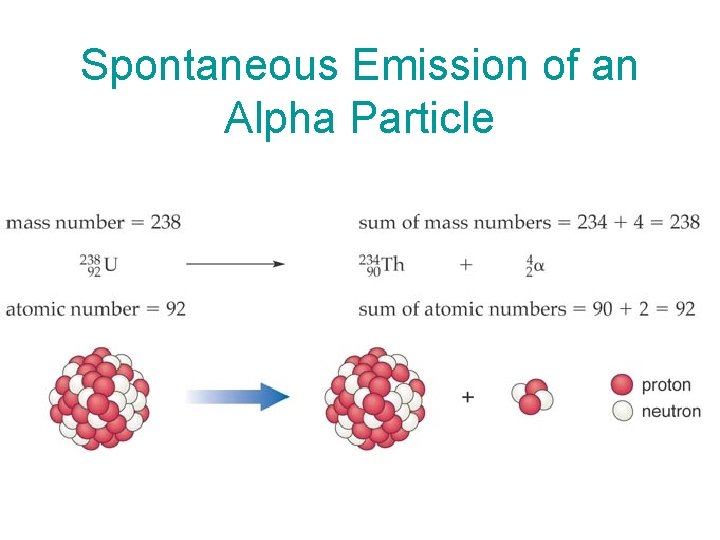
Alpha emission: a type of nuclear decay in which a helium nucleus (2 protons + 2 neutrons) is emitted from the nucleus. In alpha emission, the new element formed has an atomic number two units lower and a mass number four units lower than the original nucleus. I’m an alpha particle!!!

Spontaneous Emission of an Alpha Particle

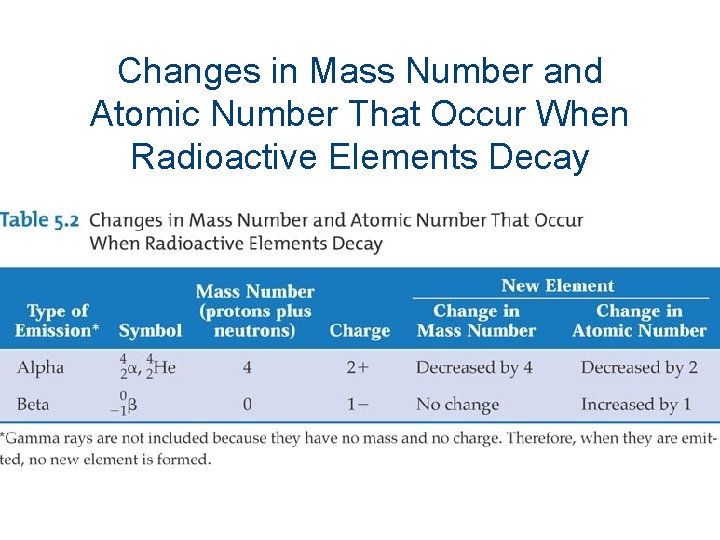
Changes in Mass Number and Atomic Number That Occur When Radioactive Elements Decay

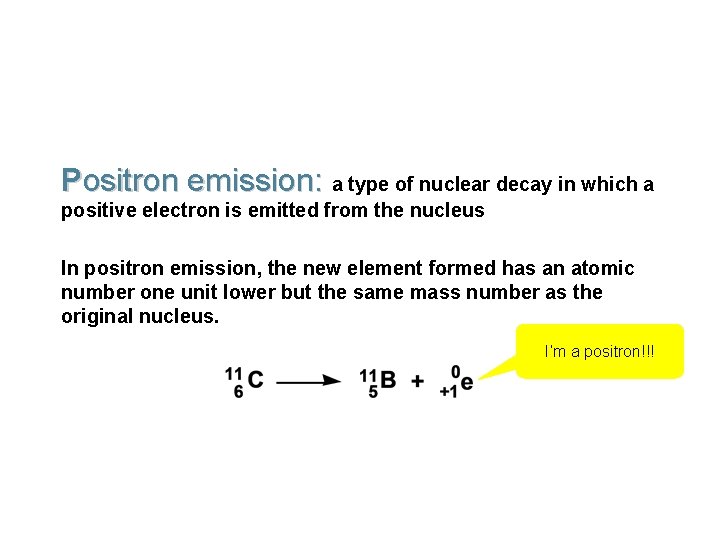
Positron emission: a type of nuclear decay in which a positive electron is emitted from the nucleus In positron emission, the new element formed has an atomic number one unit lower but the same mass number as the original nucleus. I’m a positron!!!

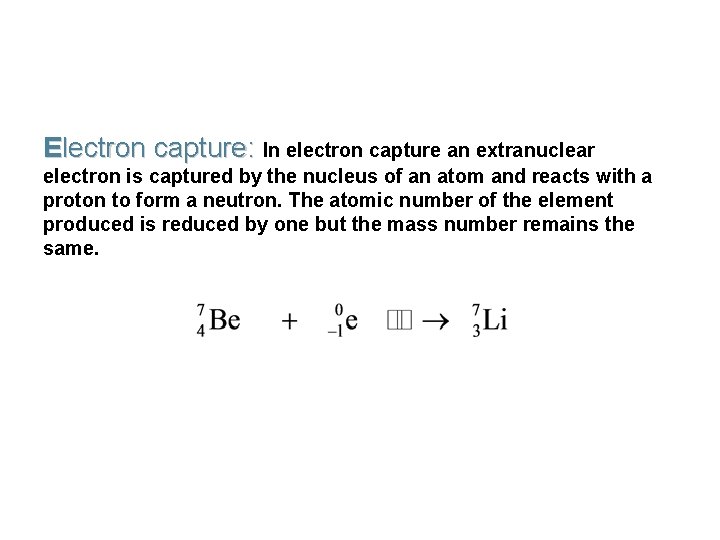
Electron capture: In electron capture an extranuclear electron is captured by the nucleus of an atom and reacts with a proton to form a neutron. The atomic number of the element produced is reduced by one but the mass number remains the same.

Nuclear Radiation There are more than 300 naturally occurring isotopes – 264 are stable (not radioactive) and the remainder are radioactive. – among the lighter elements, stable isotopes have approximately the same number of protons and neutrons: 126 C, 168 O, and 2010 Ne – among the heavier elements, stability requires more neutrons than protons; the most stable isotope of lead, is lead-206, 12482 Pb More than 1000 artificial isotopes have been made in the laboratory; all are radioactive

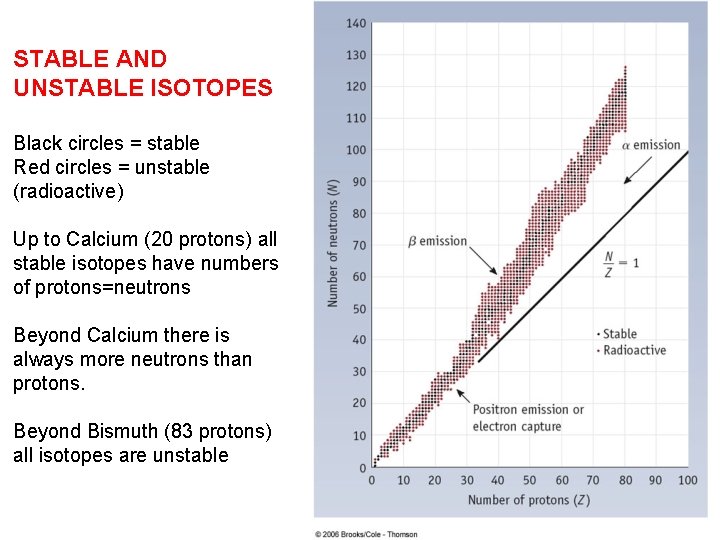
STABLE AND UNSTABLE ISOTOPES Black circles = stable Red circles = unstable (radioactive) Up to Calcium (20 protons) all stable isotopes have numbers of protons=neutrons Beyond Calcium there is always more neutrons than protons. Beyond Bismuth (83 protons) all isotopes are unstable

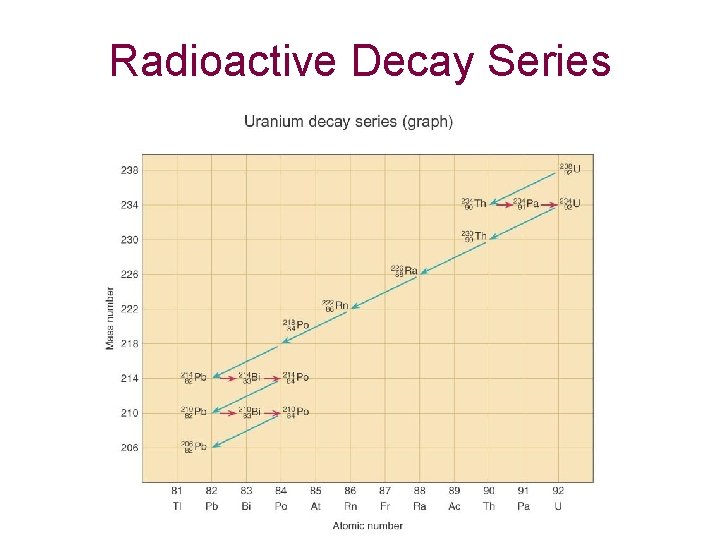
Radioactive Decay Series

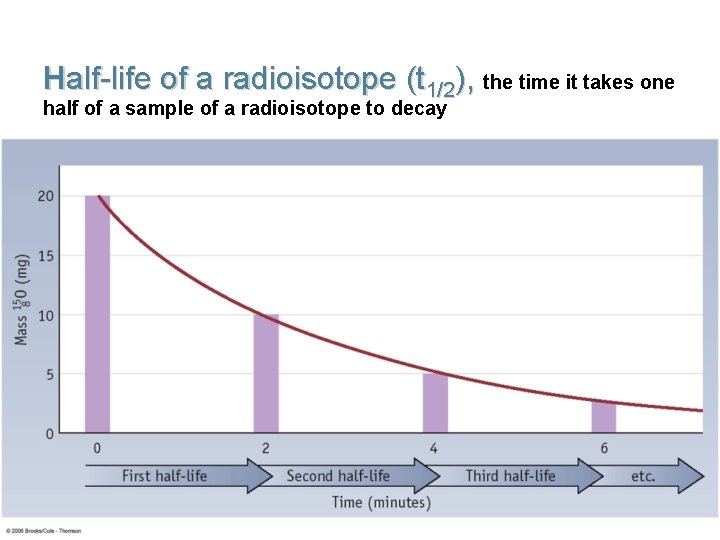
Half-life of a radioisotope (t 1/2), the time it takes one half of a sample of a radioisotope to decay

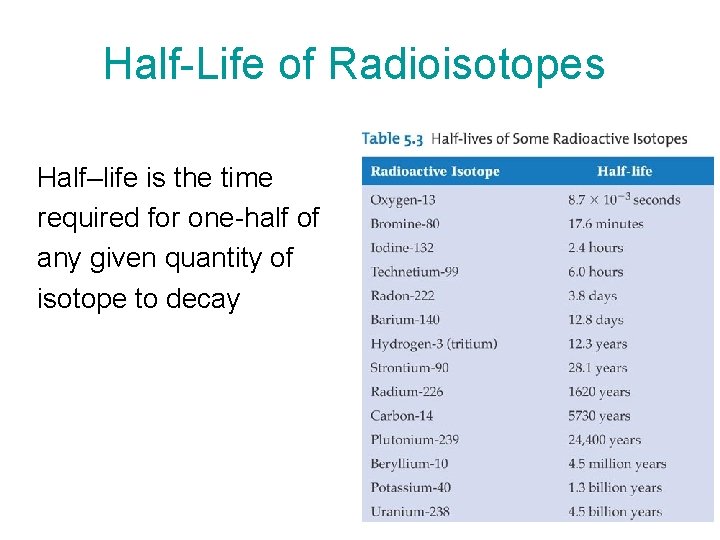
Half-Life of Radioisotopes Half–life is the time required for one-half of any given quantity of isotope to decay

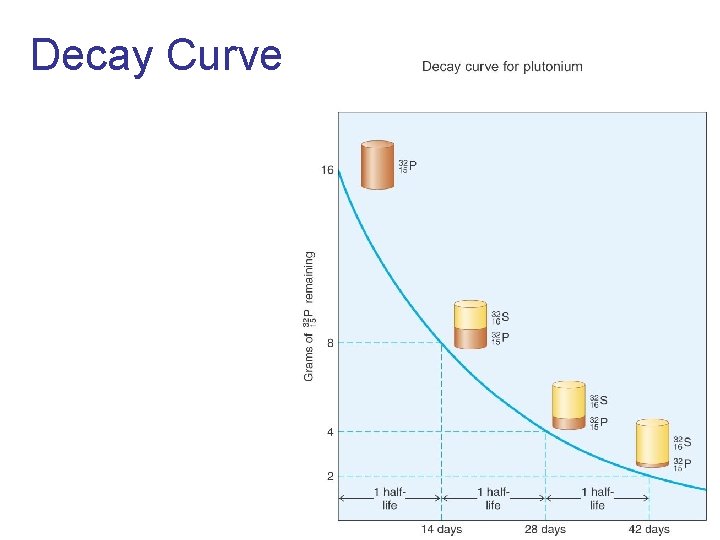
Decay Curve


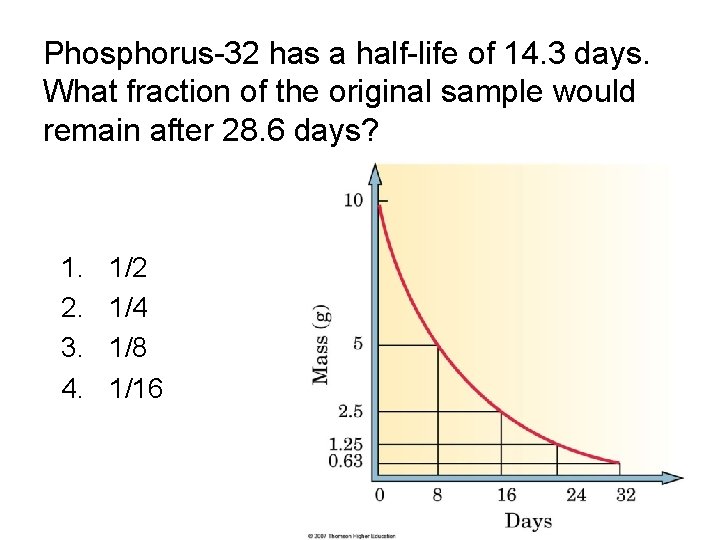
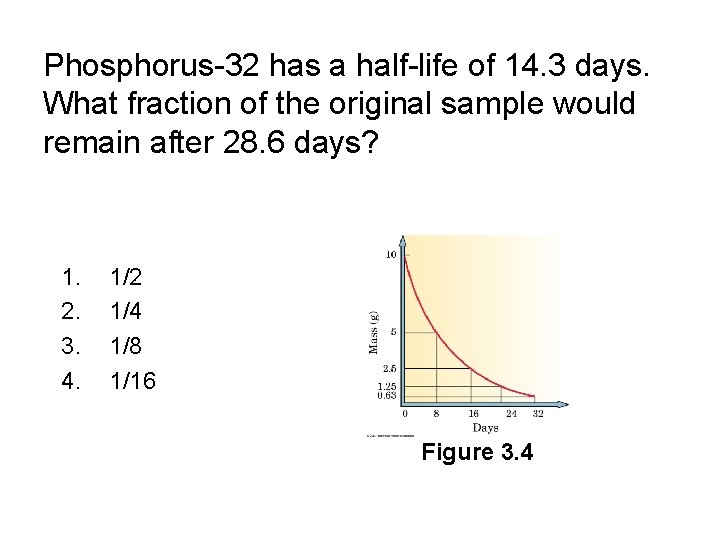
Phosphorus-32 has a half-life of 14. 3 days. What fraction of the original sample would remain after 28. 6 days? 1. 2. 3. 4. 1/2 1/4 1/8 1/16

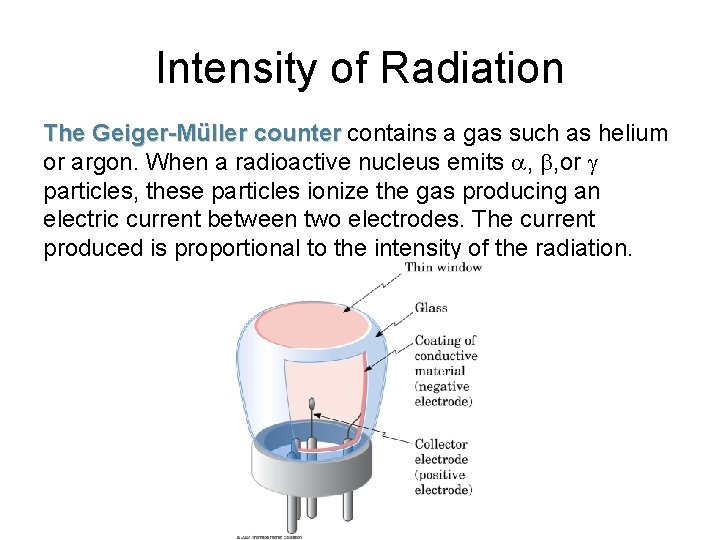
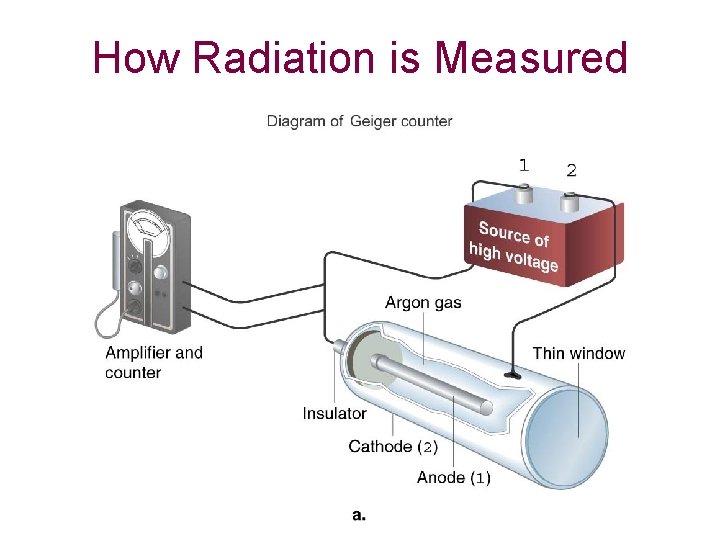
Intensity of Radiation The Geiger-Müller counter contains a gas such as helium or argon. When a radioactive nucleus emits a, b, or g particles, these particles ionize the gas producing an electric current between two electrodes. The current produced is proportional to the intensity of the radiation.

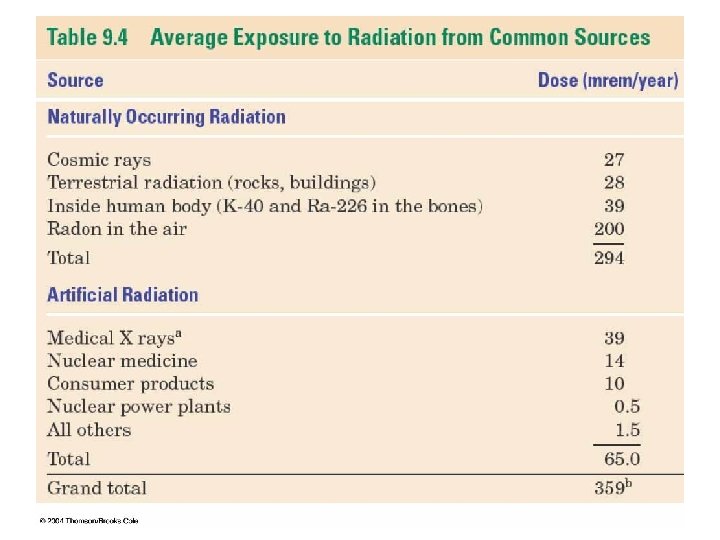
How Radiation is Measured

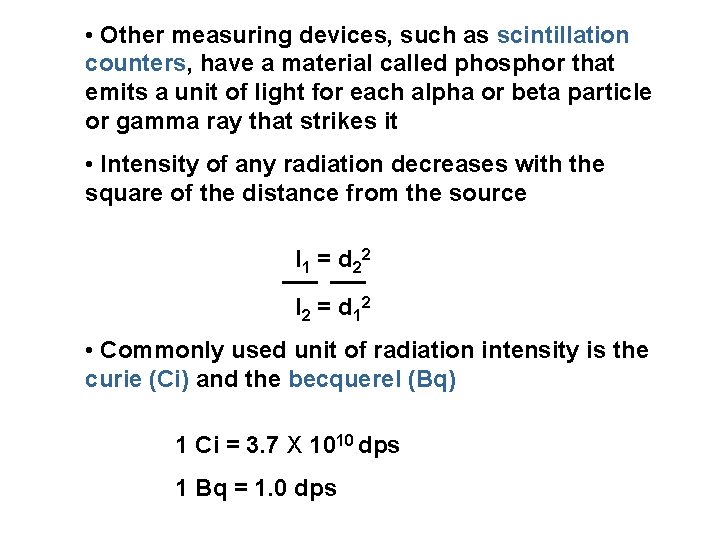
• Other measuring devices, such as scintillation counters, have a material called phosphor that emits a unit of light for each alpha or beta particle or gamma ray that strikes it • Intensity of any radiation decreases with the square of the distance from the source I 1 = d 22 I 2 = d 12 • Commonly used unit of radiation intensity is the curie (Ci) and the becquerel (Bq) 1 Ci = 3. 7 X 1010 dps 1 Bq = 1. 0 dps

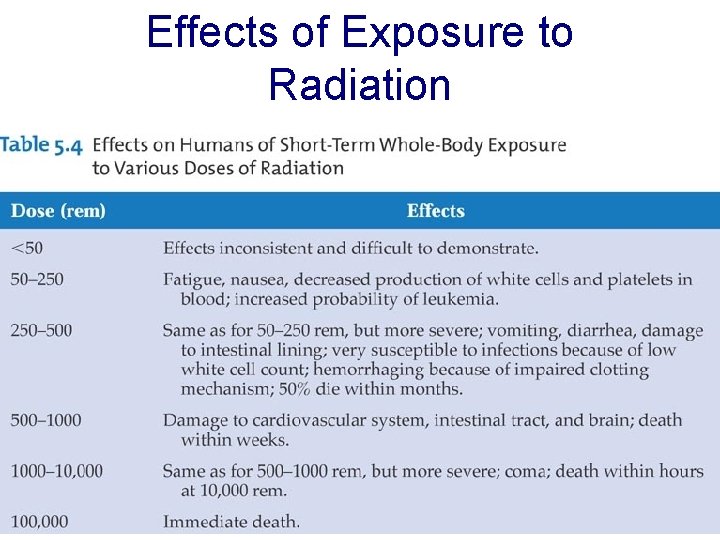
Effects of Exposure to Radiation


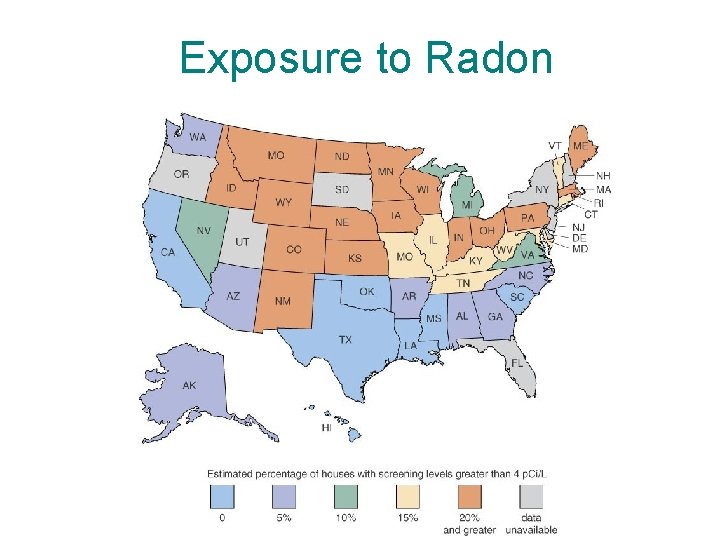
Exposure to Radon

Plants incorporate C-14 into living tissue. The percentage of C-14 in the plant is the same as in the atmosphere. When the plant dies C-14 is no longer incorporated but radioactive decay continues with C-14 activity decreasing over time. A plant that has recently died will emit 13. 7 dpm/g of carbon. After 5370 years it will emit about 7 dpm and after 11460 years about 3. 5 dpm. Humans incorporate C-14 through their diet while alive. After death C-14 is no longer incorporated. Only decay takes place. The ice man, lived 5300 years ago. Found in a glacier in the Alps

Phosphorus-32 has a half-life of 14. 3 days. What fraction of the original sample would remain after 28. 6 days? 1. 2. 3. 4. 1/2 1/4 1/8 1/16 Figure 3. 4

Using the Half-lives of Radioisotopes

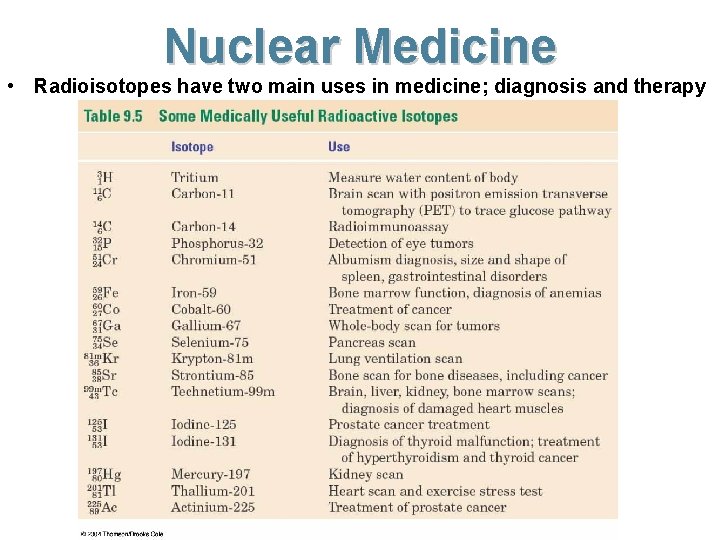
Nuclear Medicine • Radioisotopes have two main uses in medicine; diagnosis and therapy

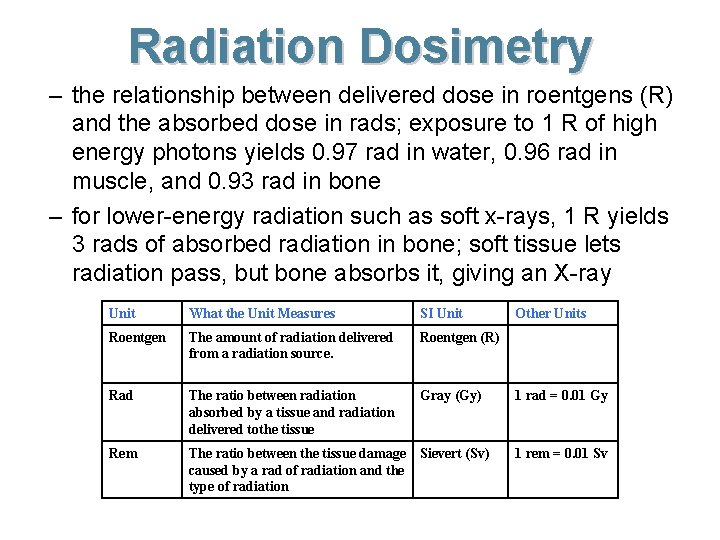
Radiation Dosimetry – the relationship between delivered dose in roentgens (R) and the absorbed dose in rads; exposure to 1 R of high energy photons yields 0. 97 rad in water, 0. 96 rad in muscle, and 0. 93 rad in bone – for lower-energy radiation such as soft x-rays, 1 R yields 3 rads of absorbed radiation in bone; soft tissue lets radiation pass, but bone absorbs it, giving an X-ray Unit What the Unit Measures SI Unit Roentgen The amount of radiation delivered from a radiation source. Roentgen (R) Rad The ratio between radiation absorbed by a tissue and radiation delivered tothe tissue Gray (Gy) Rem The ratio between the tissue damage Sievert (Sv) caused by a rad of radiation and the type of radiation Other Units 1 rad = 0. 01 Gy 1 rem = 0. 01 Sv

– a single whole-body irradiation of 25 rem is noticeable in blood count – a single dose of 100 rem causes typical symptoms of radiation sickness – a single dose of 400 rem causes death within one month in 50% of the exposed persons – a single dose of 600 rem is almost invariably lethal within a month – it is estimated that a single dose of 50, 000 rem is needed to kill bacteria, and up to 106 rem is needed to inactivate viruses

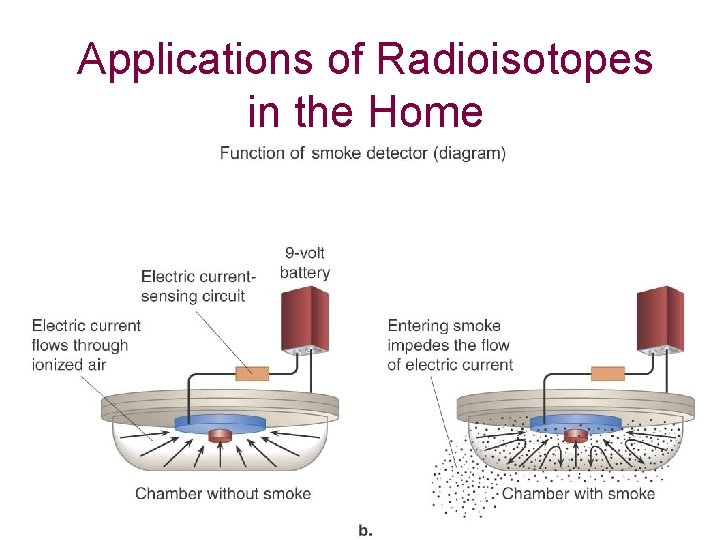
Applications of Radioisotopes in the Home

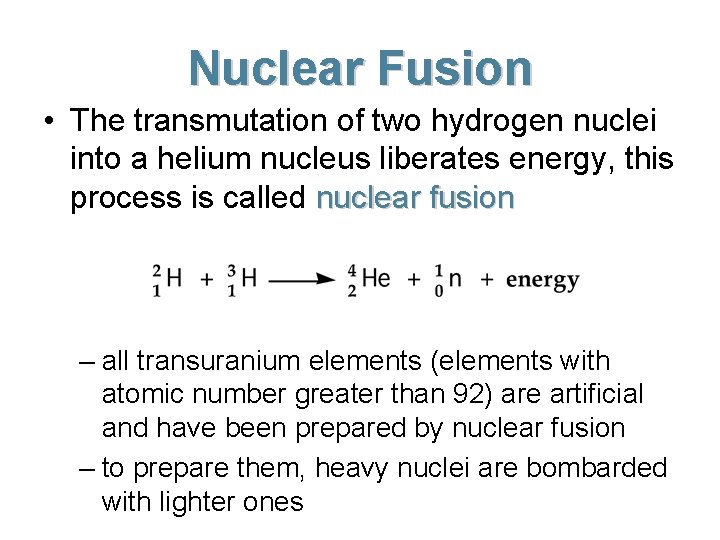
Nuclear Fusion • The transmutation of two hydrogen nuclei into a helium nucleus liberates energy, this process is called nuclear fusion – all transuranium elements (elements with atomic number greater than 92) are artificial and have been prepared by nuclear fusion – to prepare them, heavy nuclei are bombarded with lighter ones

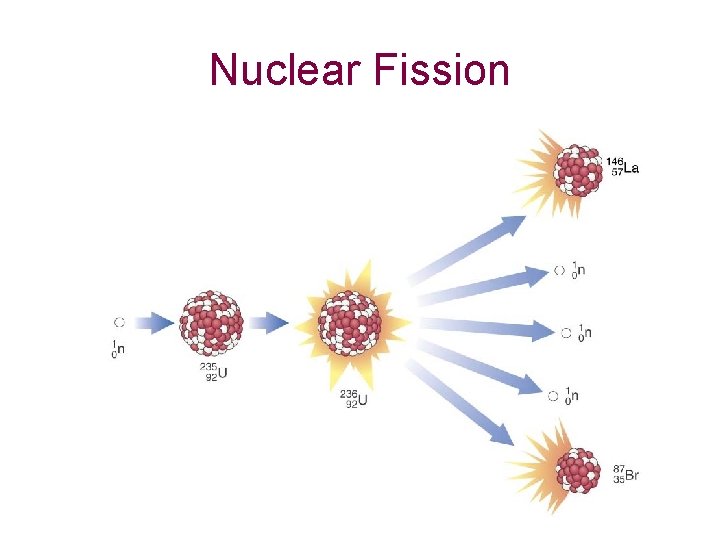
Nuclear Fission

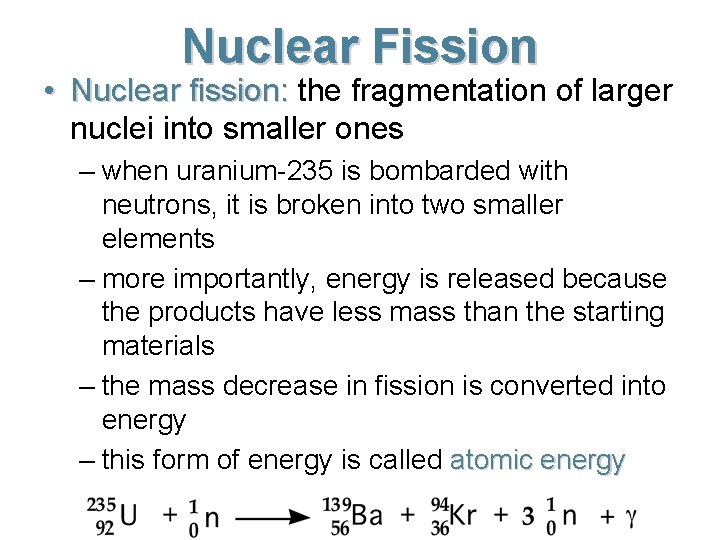
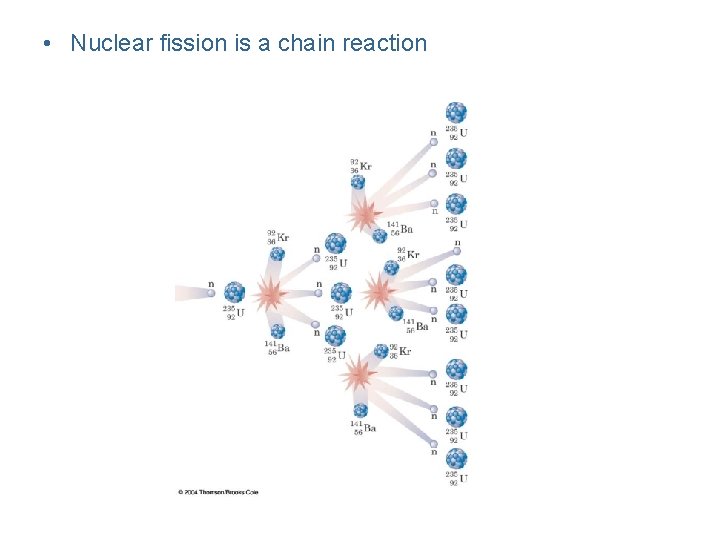
Nuclear Fission • Nuclear fission: the fragmentation of larger nuclei into smaller ones – when uranium-235 is bombarded with neutrons, it is broken into two smaller elements – more importantly, energy is released because the products have less mass than the starting materials – the mass decrease in fission is converted into energy – this form of energy is called atomic energy

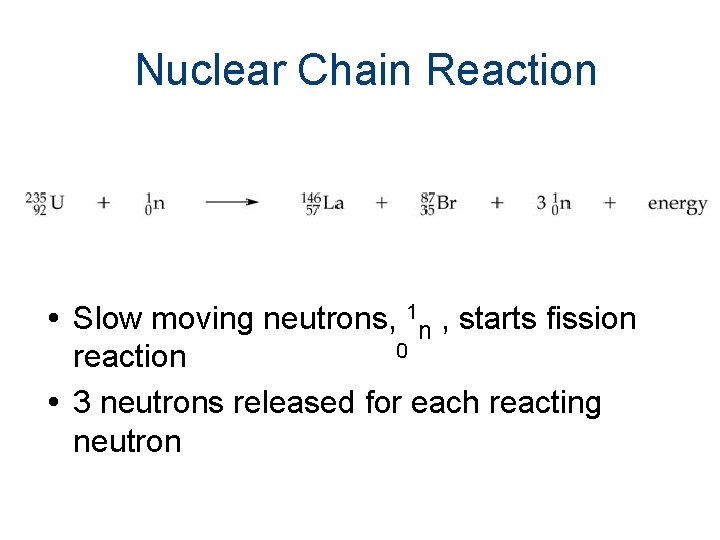
Nuclear Chain Reaction Slow moving neutrons, 1 n , starts fission 0 reaction 3 neutrons released for each reacting neutron

• Nuclear fission is a chain reaction

Energy Released During Nuclear Fission Einstein predicted: E=mc 2 C (speed of light) = 300, 000 km/sec or = 186, 000 miles/sec Even a small amount of mass when multiplied by c 2 will produce an enormous amount of energy.

Nuclear Fission – today more than 15% of the electrical energy in the United States is supplied by nuclear power plants – disposal of spent but still radioactive fuel materials is a major long-term problem – spent fuel contains high-level fission products together with recoverable uranium and plutonium – in addition, there are radioactive wastes from nuclear weapons programs, research reactors, and so forth

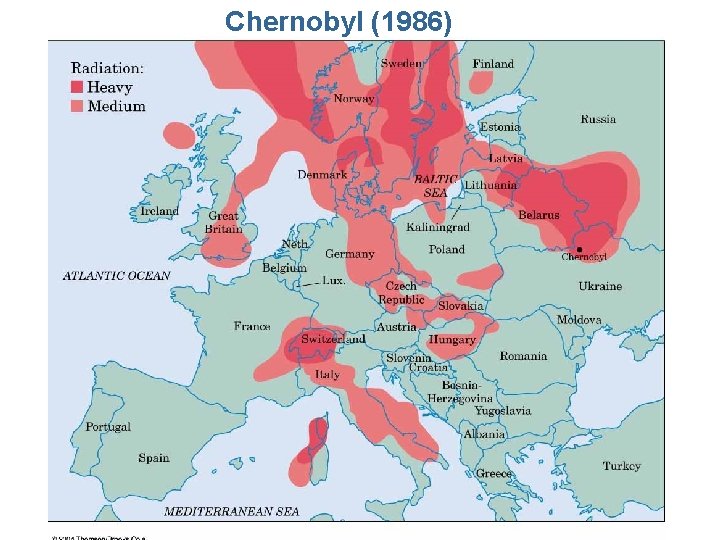
Chernobyl (1986)

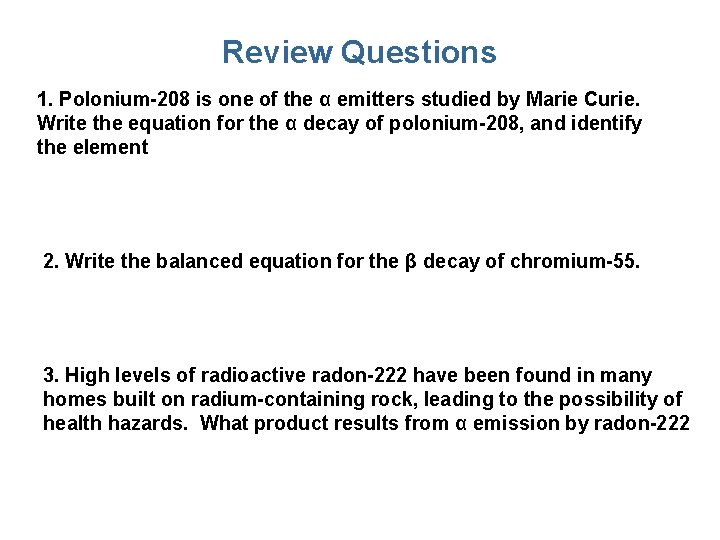
Review Questions 1. Polonium-208 is one of the α emitters studied by Marie Curie. Write the equation for the α decay of polonium-208, and identify the element 2. Write the balanced equation for the β decay of chromium-55. 3. High levels of radioactive radon-222 have been found in many homes built on radium-containing rock, leading to the possibility of health hazards. What product results from α emission by radon-222

Review Questions 4. Carbon-14, a β emitter, is a rare isotope used in dating archaeological artifacts. Write a nuclear equation for the decay of carbon-14. 5. Phosphorus-32, a radioisotope used in leukemia therapy, has a half-life of about 14 days. Approximately what percent of a sample remains after 8 weeks? 6. Selenium-75, a β emitter with a half-life of 120 days, is used medically for pancreas scans. Approximately how much selenium-75 would remain from a 12. 0 g sample that has been stored for 1 year?

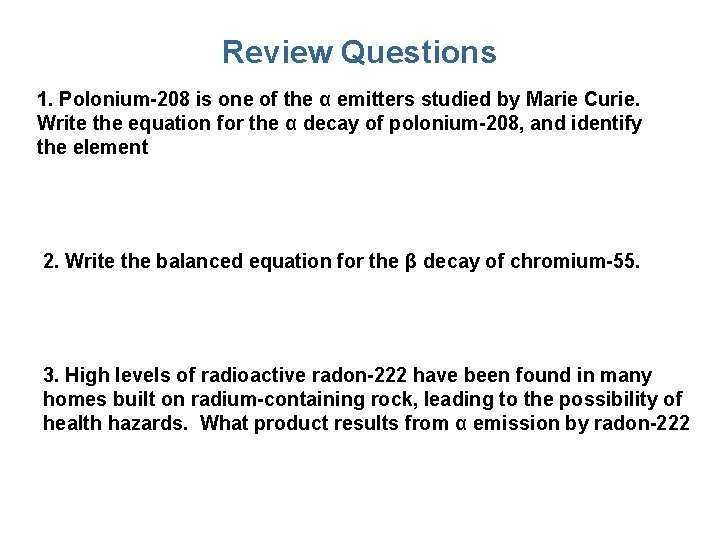
Review Questions 1. Polonium-208 is one of the α emitters studied by Marie Curie. Write the equation for the α decay of polonium-208, and identify the element 2. Write the balanced equation for the β decay of chromium-55. 3. High levels of radioactive radon-222 have been found in many homes built on radium-containing rock, leading to the possibility of health hazards. What product results from α emission by radon-222

Review Questions 4. Carbon-14, a β emitter, is a rare isotope used in dating archaeological artifacts. Write a nuclear equation for the decay of carbon-14. 5. Write a balanced nuclear equation for electron capture by polonium-204. 6. Write a balanced nuclear equation for the positron emission from xenon-118.

Review Questions 7. Phosphorus-32, a radioisotope used in leukemia therapy, has a half-life of about 14 days. Approximately what percent of a sample remains after 8 weeks? 8. Selenium-75, a β emitter with a half-life of 120 days, is used medically for pancreas scans. Approximately how much selenium-75 would remain from a 0. 050 g sample that has been stored for 1 year? 9. If a radiation source give 75 units of radiation at a distance of 2. 4 m, at what distance would the source give 25 units of radiation?