The Nature of Matter Section 2 1 Atoms

- Slides: 13

The Nature of Matter Section 2. 1

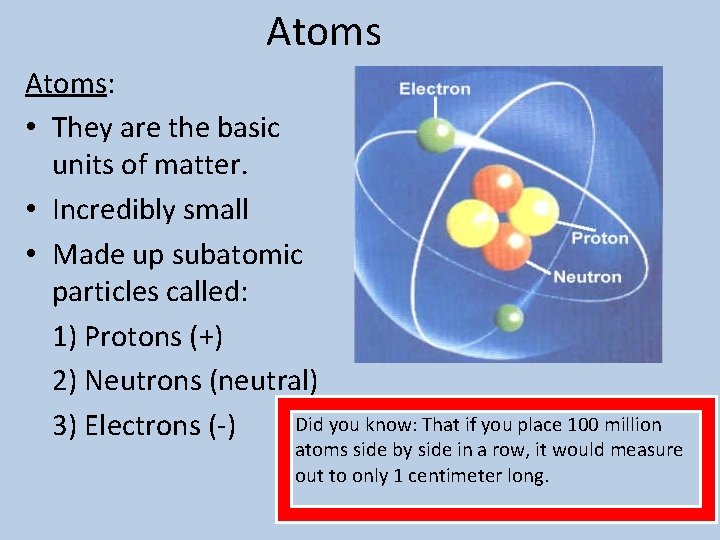
Atoms: • They are the basic units of matter. • Incredibly small • Made up subatomic particles called: 1) Protons (+) 2) Neutrons (neutral) Did you know: That if you place 100 million 3) Electrons (-) atoms side by side in a row, it would measure out to only 1 centimeter long.

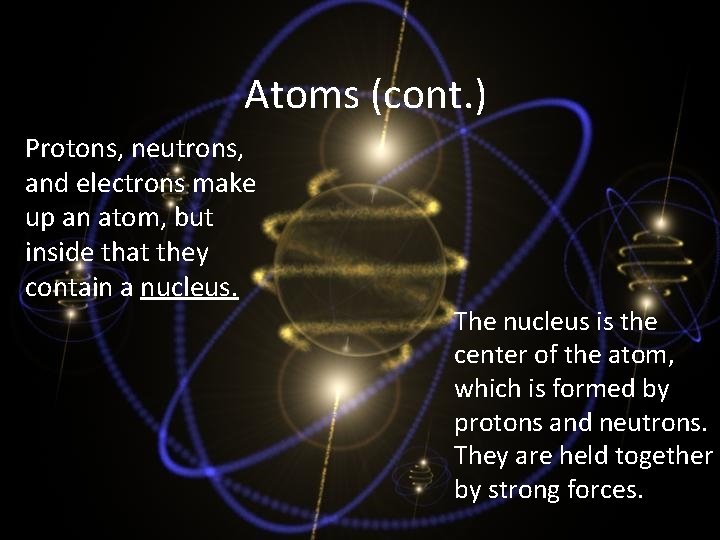
Atoms (cont. ) Protons, neutrons, and electrons make up an atom, but inside that they contain a nucleus. The nucleus is the center of the atom, which is formed by protons and neutrons. They are held together by strong forces.

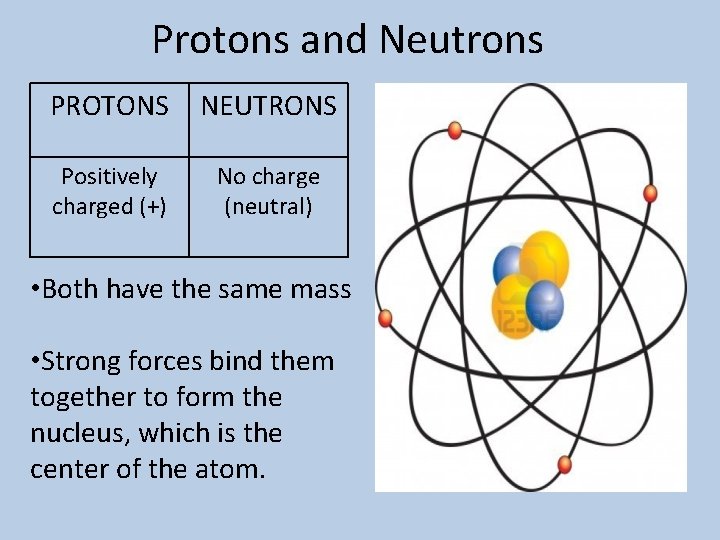
Protons and Neutrons PROTONS NEUTRONS Positively charged (+) No charge (neutral) • Both have the same mass • Strong forces bind them together to form the nucleus, which is the center of the atom.

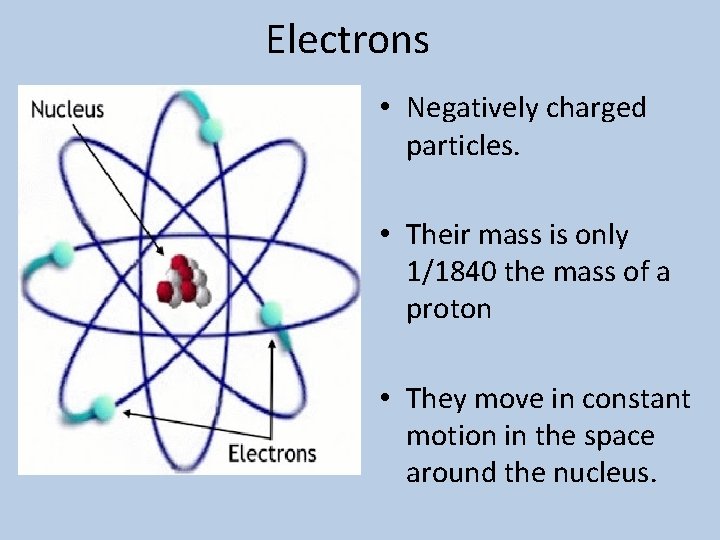
Electrons • Negatively charged particles. • Their mass is only 1/1840 the mass of a proton • They move in constant motion in the space around the nucleus.

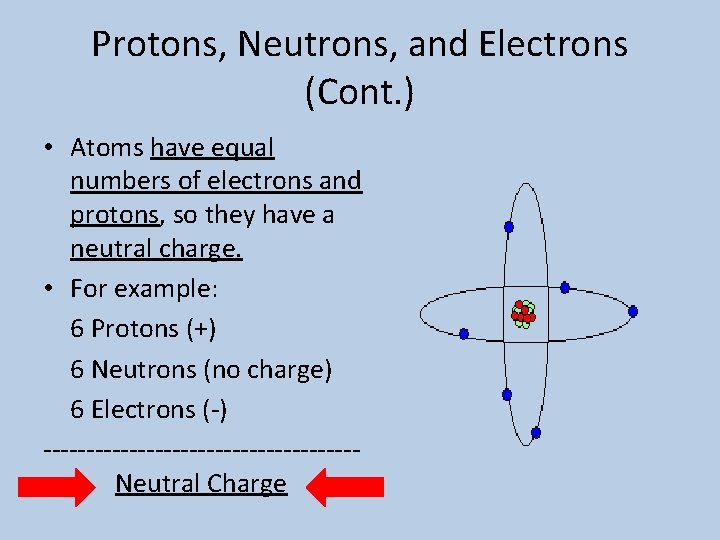
Protons, Neutrons, and Electrons (Cont. ) • Atoms have equal numbers of electrons and protons, so they have a neutral charge. • For example: 6 Protons (+) 6 Neutrons (no charge) 6 Electrons (-) ------------------Neutral Charge

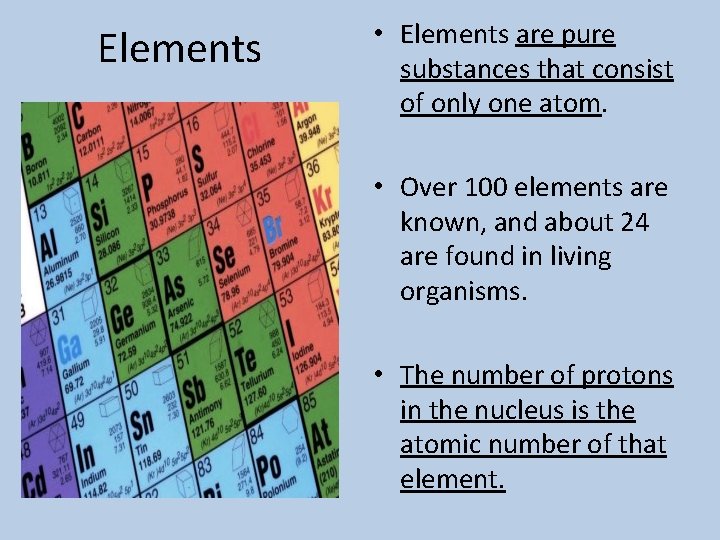
Elements • Elements are pure substances that consist of only one atom. • Over 100 elements are known, and about 24 are found in living organisms. • The number of protons in the nucleus is the atomic number of that element.

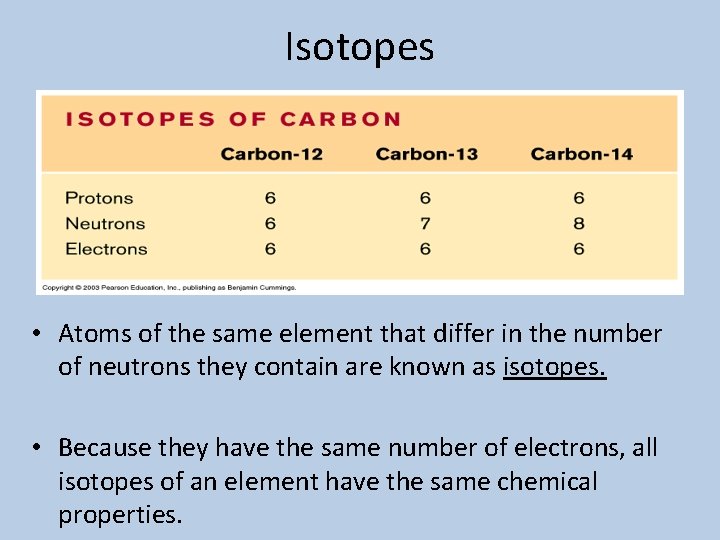
Isotopes • Atoms of the same element that differ in the number of neutrons they contain are known as isotopes. • Because they have the same number of electrons, all isotopes of an element have the same chemical properties.

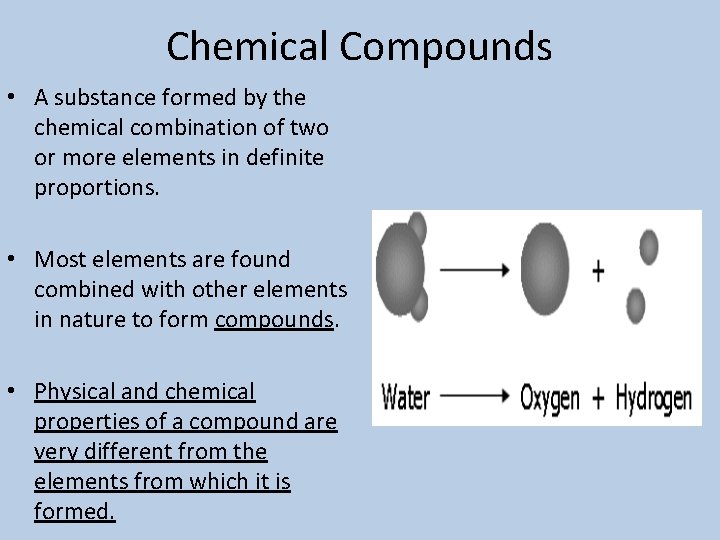
Chemical Compounds • A substance formed by the chemical combination of two or more elements in definite proportions. • Most elements are found combined with other elements in nature to form compounds. • Physical and chemical properties of a compound are very different from the elements from which it is formed.

Chemical Bonds • Bond formation involves the electrons that surround each atomic nucleus. • Atoms in compounds are held together by various chemical bonds, but the main ones are IONIC and COVALENT bonds.

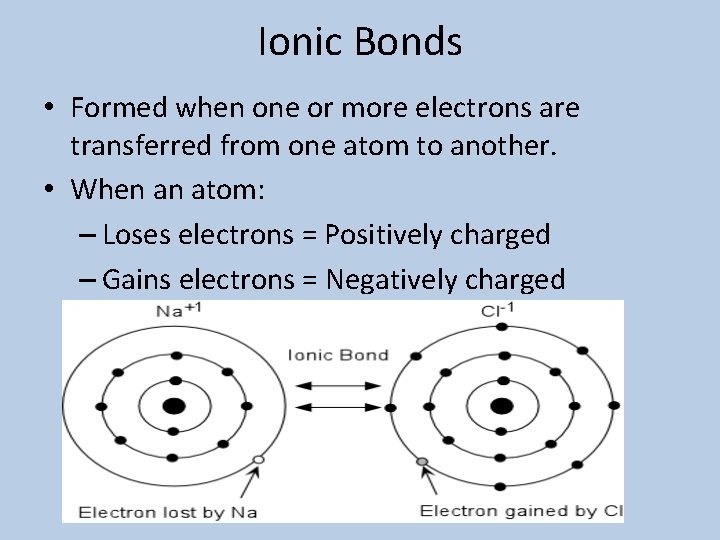
Ionic Bonds • Formed when one or more electrons are transferred from one atom to another. • When an atom: – Loses electrons = Positively charged – Gains electrons = Negatively charged

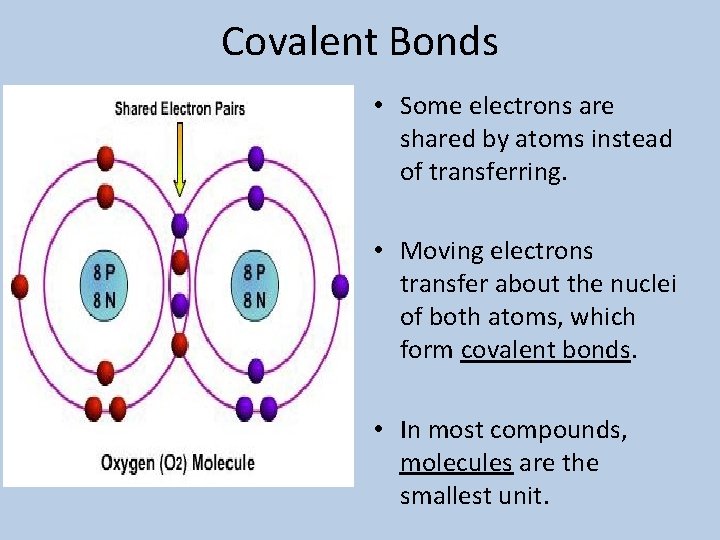
Covalent Bonds • Some electrons are shared by atoms instead of transferring. • Moving electrons transfer about the nuclei of both atoms, which form covalent bonds. • In most compounds, molecules are the smallest unit.

Van der Waals Forces • When molecules are close, a slight attraction can develop between the oppositely charged regions of nearby molecules. • Chemists call this intermolecular forces of attraction van der waals forces.