The Nature of Matter Atoms and Bonding Atoms

The Nature of Matter Atoms and Bonding
Atoms • Atoms – The basic unit of matter.

Structure of an Atom Proton Electron Neutron Nucleus

Your Turn! • Describe each of the subatomic particles in your own words. – Nucleus – Proton – Neutron – Electron
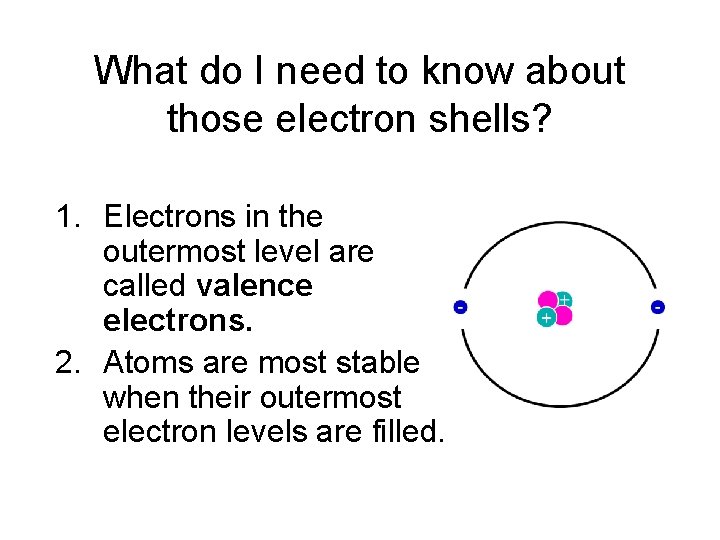
What do I need to know about those electron shells? 1. Electrons in the outermost level are called valence electrons. 2. Atoms are most stable when their outermost electron levels are filled.
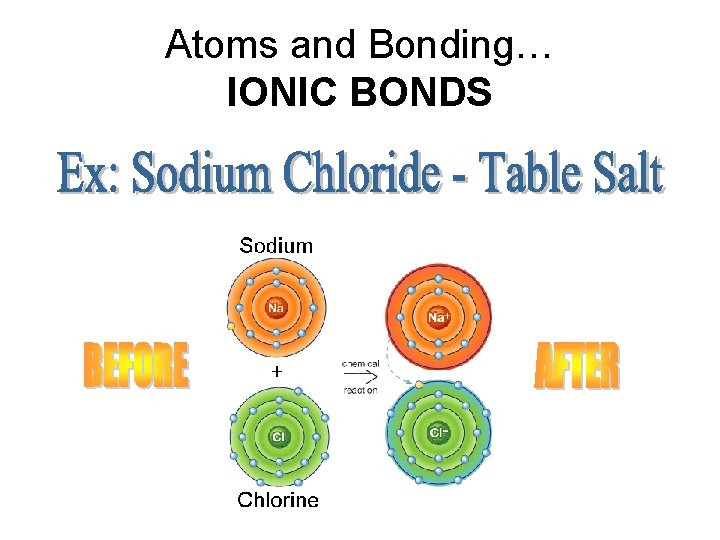
Atoms and Bonding… IONIC BONDS
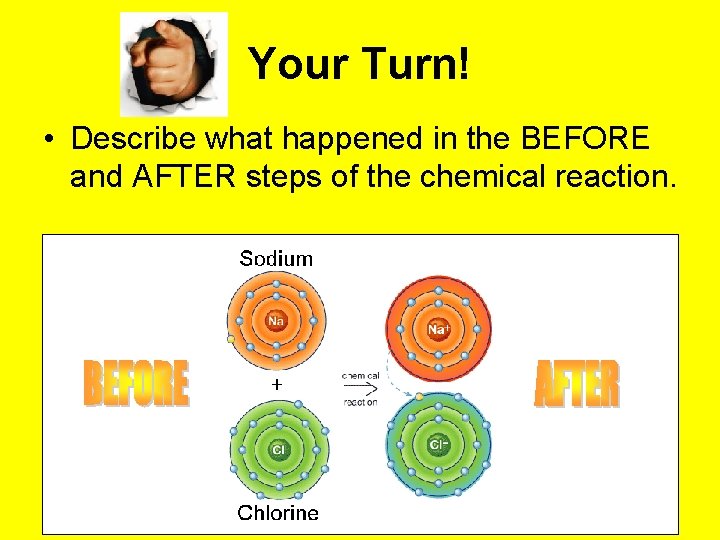
Your Turn! • Describe what happened in the BEFORE and AFTER steps of the chemical reaction.
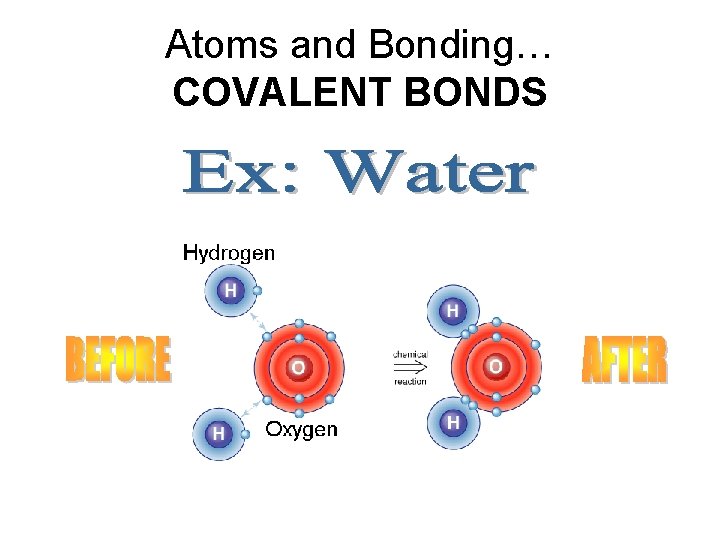
Atoms and Bonding… COVALENT BONDS
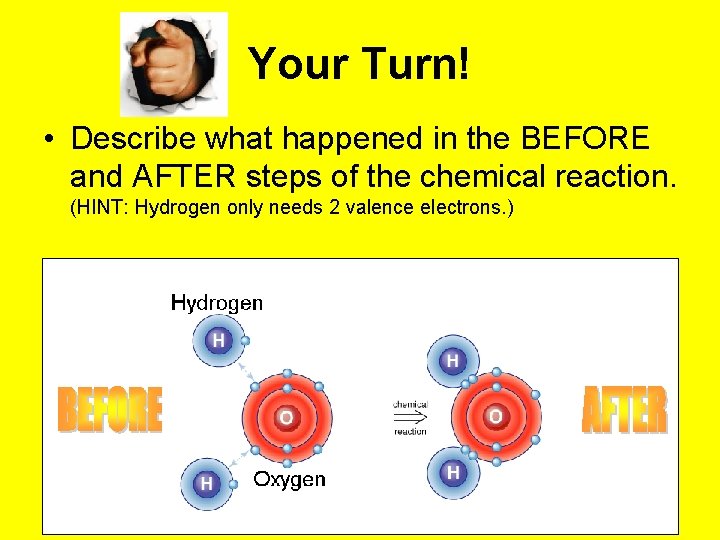
Your Turn! • Describe what happened in the BEFORE and AFTER steps of the chemical reaction. (HINT: Hydrogen only needs 2 valence electrons. )

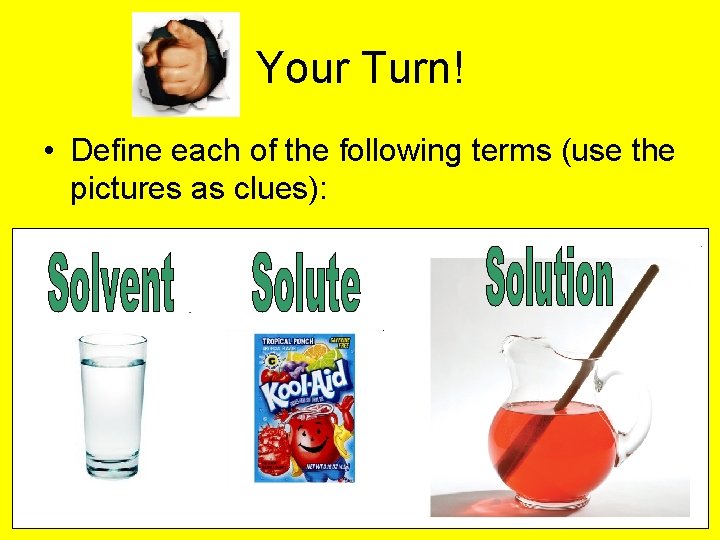
Your Turn! • Define each of the following terms (use the pictures as clues):
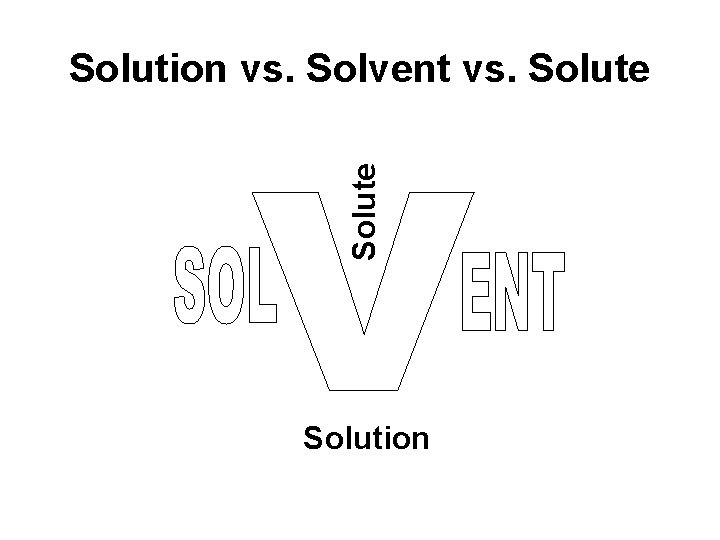
Solute Solution vs. Solvent vs. Solute Solution

Solution vs. Solvent vs. Solute • Water is known as the universal solvent.

Your Turn! • Purpose: To determine which substances will mix with water, and to determine whether a solution is polar or nonpolar.
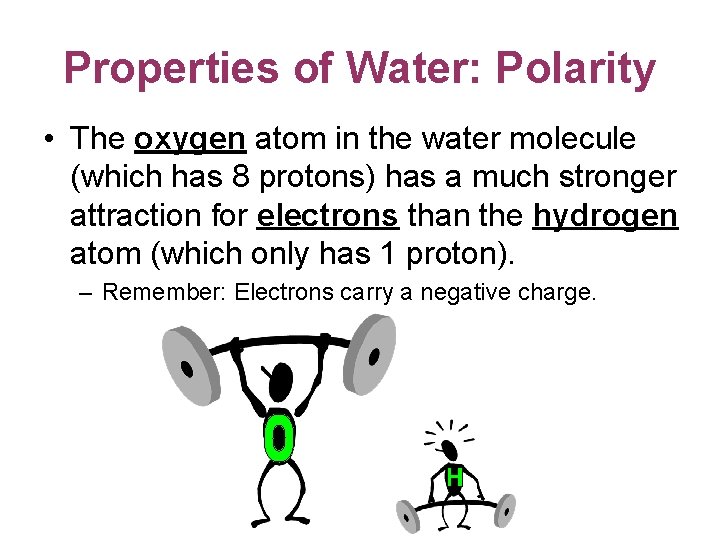
Properties of Water: Polarity • The oxygen atom in the water molecule (which has 8 protons) has a much stronger attraction for electrons than the hydrogen atom (which only has 1 proton). – Remember: Electrons carry a negative charge.
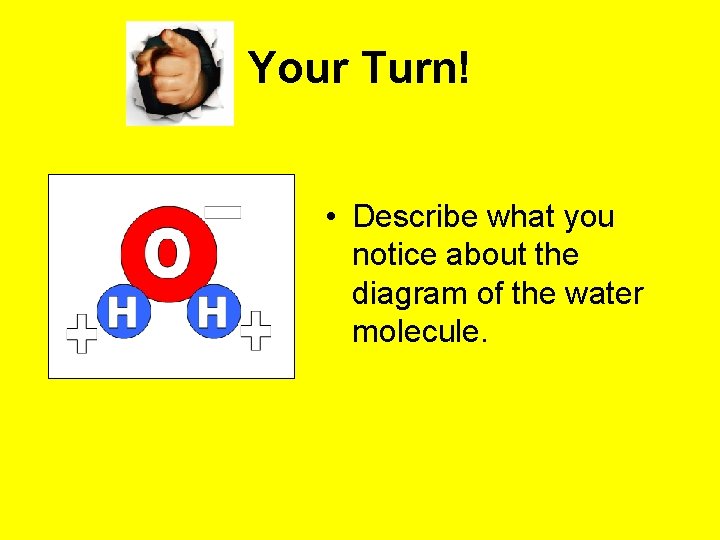
Your Turn! • Describe what you notice about the diagram of the water molecule.
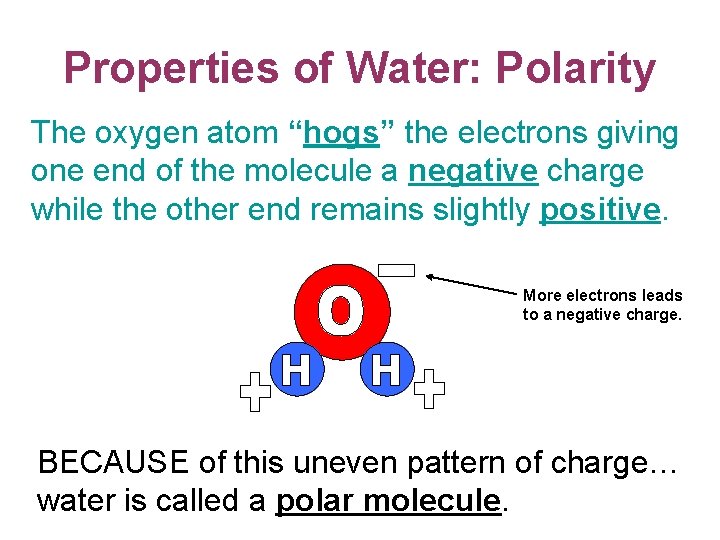
Properties of Water: Polarity The oxygen atom “hogs” the electrons giving one end of the molecule a negative charge while the other end remains slightly positive. More electrons leads to a negative charge. BECAUSE of this uneven pattern of charge… water is called a polar molecule.

Your Turn! • What is a polar molecule?

What’s so special about being a polar compound? • • It makes water VERY good at dissolving many other substances. It can dissolve other polar substances like sugars, as well as ionic compounds (compounds with a charge) like sodium chloride (salt).
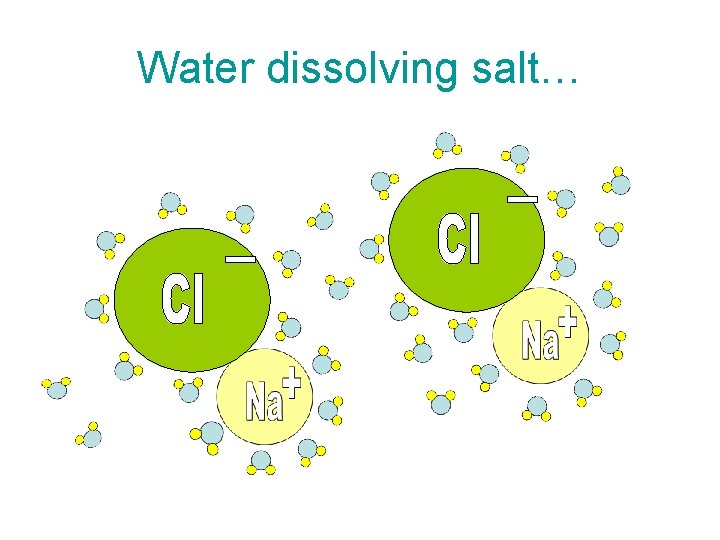
Water dissolving salt…
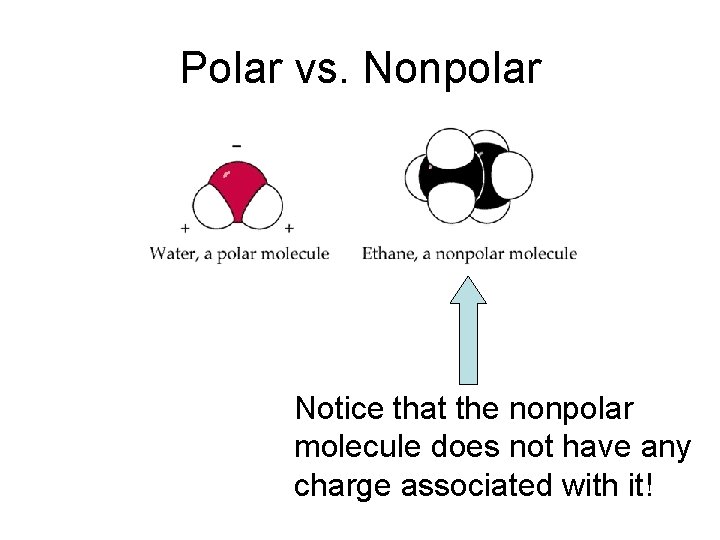
Polar vs. Nonpolar Notice that the nonpolar molecule does not have any charge associated with it!
Your Turn! • What is a nonpolar molecule? • What did you learn in the lab about how polar and nonpolar substances interact?

Your Turn! • Purpose: To observe the movement of water through capillary action and to observe how this property of water can be used to separate a mixture of substances into the mixture’s individual components. • Purpose: To observe the effects of the attractive force between water molecules.
Your Turn! • Watch the water animation to define and describe a hydrogen bond.
Hydrogen bonding creates… Cohesion • The attractive forces between particles of the same kind (water to water). Adhesion • The attractive force between unlike substances (water to penny).
Your Turn! • Why does a piece of celery sitting in blue water gradually turn blue? • Use the following vocabulary words in your answer: – Adhesion – Cohesion – Capillary Action
Surface Tension • The measure of how difficult it is to break the surface of a liquid. • Water has very high surface tension which can create a “skin” across the surface of water.

Additional Properties of Water: Heat Capacity • Water must gain or lose a large amount of energy for its temperature to change. – The energy needs to first break the hydrogen bonds before it can raise the temperature of the water. • Water has a high heat capacity!
Your Turn! • Why do you think it is important to us that water has a high heat capacity? Homeostasis: maintaining a stable internal environment

Additional Properties of Water: Three States of Matter • Water can naturally be found in all 3 major states of matter: solid (ice), liquid (water), and gas (water vapor)

Additional Properties of Water: Density • Ice is unique because it is less dense than liquid water.

Your Turn! • Why can it be beneficial to living things that the solid form of water is less dense than the liquid form?

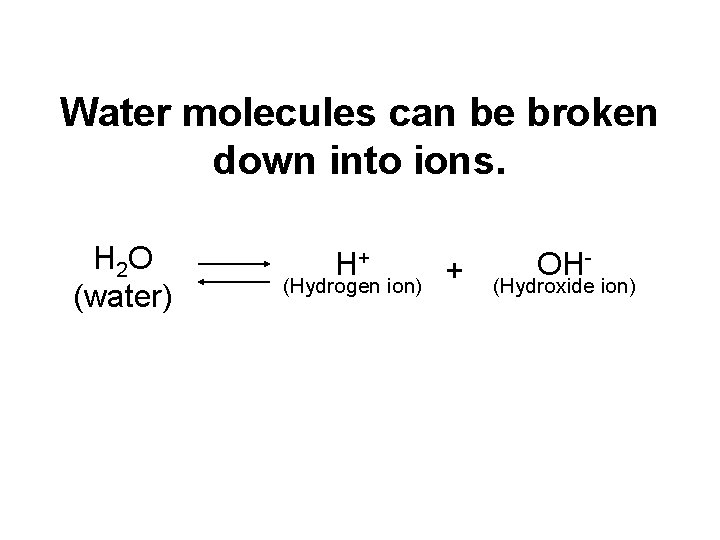
Water molecules can be broken down into ions. H 2 O (water) H+ (Hydrogen ion) + OH- (Hydroxide ion)
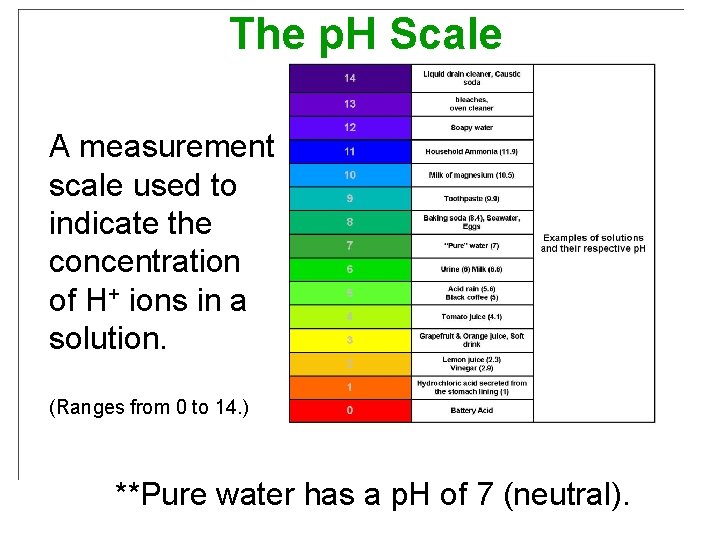
The p. H Scale A measurement scale used to indicate the concentration of H+ ions in a solution. (Ranges from 0 to 14. ) **Pure water has a p. H of 7 (neutral).

Acids • Solutions with more H+ ions than OH- ions. • p. H below 7 Bases also known as Alkalines • Solutions with more OH- ions than H+ ions. • p. H above 7

Buffers • Weak acids or bases that can react with strong acids or bases to prevent sharp, sudden changes in p. H.
- Slides: 37