The Nature of Matter Atom basic unit of

The Nature of Matter
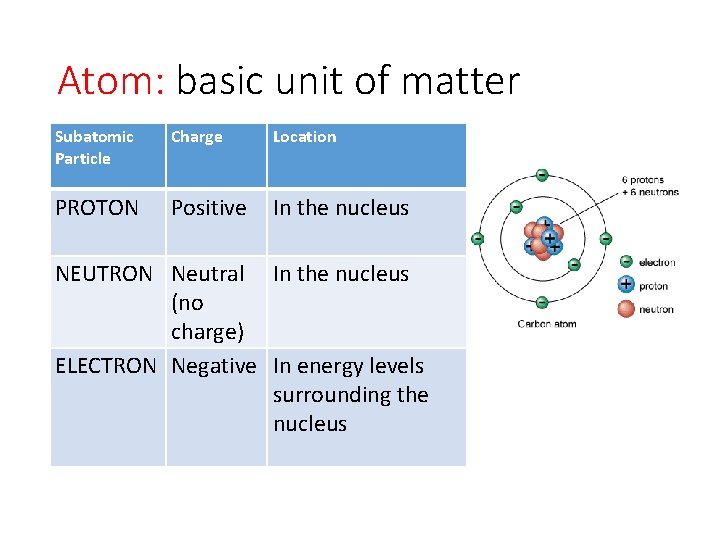
Atom: basic unit of matter Subatomic Particle Charge Location PROTON Positive In the nucleus NEUTRON Neutral In the nucleus (no charge) ELECTRON Negative In energy levels surrounding the nucleus
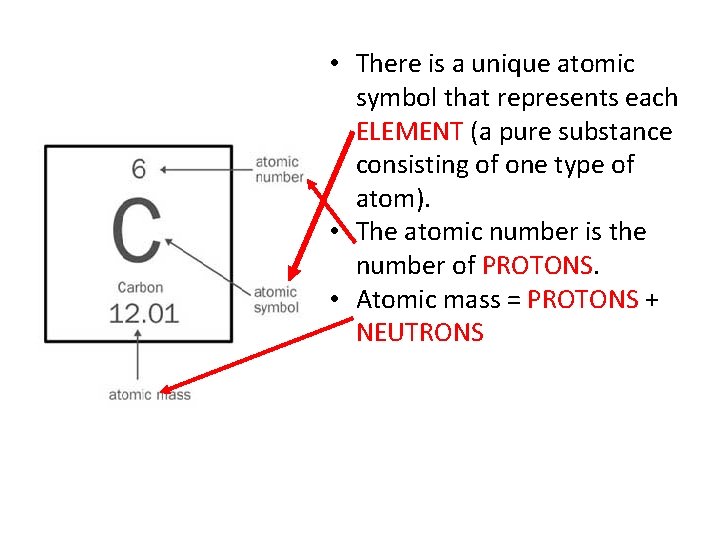
• There is a unique atomic symbol that represents each ELEMENT (a pure substance consisting of one type of atom). • The atomic number is the number of PROTONS. • Atomic mass = PROTONS + NEUTRONS
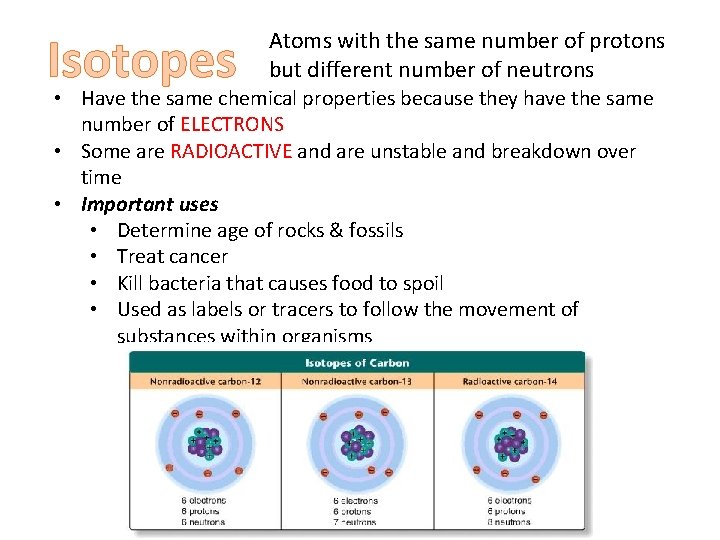
Isotopes Atoms with the same number of protons but different number of neutrons • Have the same chemical properties because they have the same number of ELECTRONS • Some are RADIOACTIVE and are unstable and breakdown over time • Important uses • Determine age of rocks & fossils • Treat cancer • Kill bacteria that causes food to spoil • Used as labels or tracers to follow the movement of substances within organisms

Compound • Substance formed by the chemical combination of two or more elements in definite proportions • Represented by a chemical formula • H 2 O, Na. Cl
Chemical Bonds • Hold together atoms in compounds • Involves the VALENCE ELECTRONS • The electrons in the outer energy level available to form bonds • Main types are ionic bonds and covalent bonds
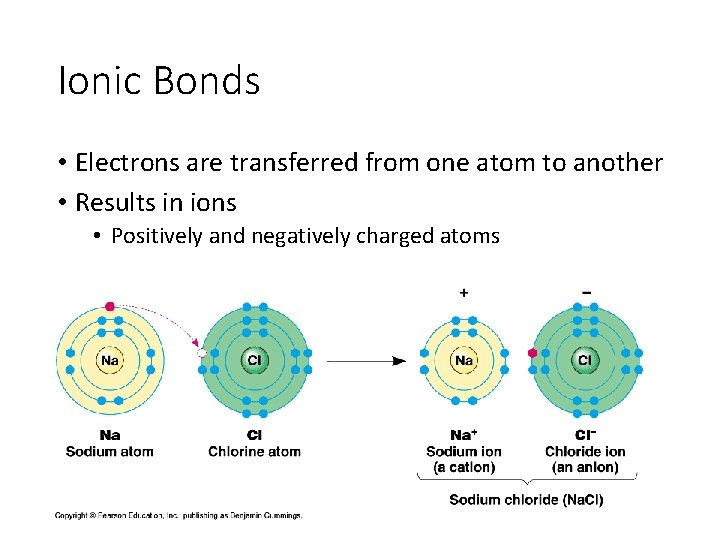
Ionic Bonds • Electrons are transferred from one atom to another • Results in ions • Positively and negatively charged atoms
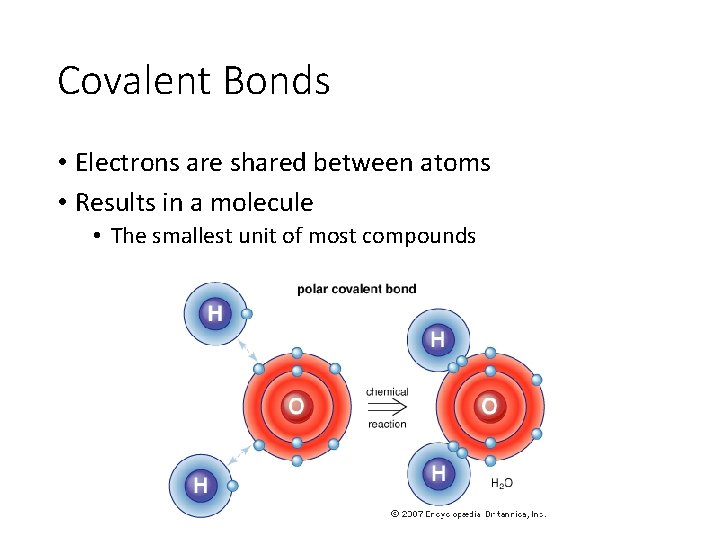
Covalent Bonds • Electrons are shared between atoms • Results in a molecule • The smallest unit of most compounds
- Slides: 8