THE NATURE OF MATERIALS 1 Crystalline Structures 2007


- Slides: 18

THE NATURE OF MATERIALS 1. Crystalline Structures © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

Crystalline Structure in which atoms are located at regular and recurring positions in three dimensions § Unit cell - basic geometric grouping of atoms that is repeated § The pattern may be replicated millions of times within a given crystal § Characteristic structure of virtually all metals, as well as many ceramics and some polymers © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

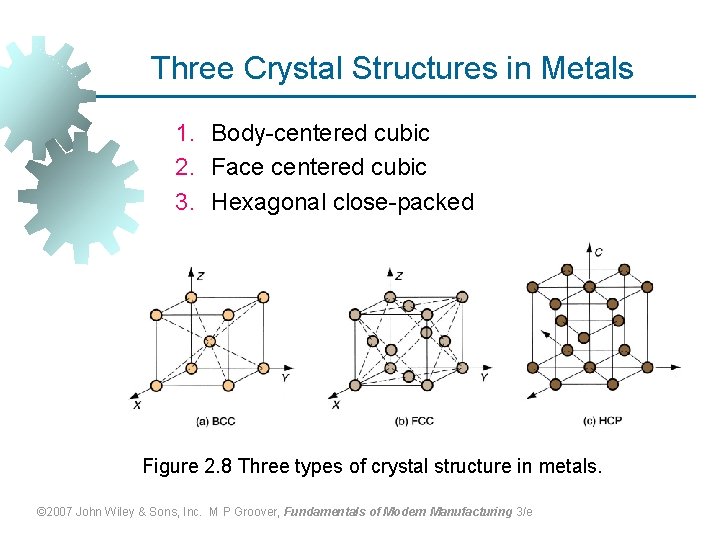
Three Crystal Structures in Metals 1. Body-centered cubic 2. Face centered cubic 3. Hexagonal close-packed Figure 2. 8 Three types of crystal structure in metals. © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

Crystal Structures for Common Metals Room temperature crystal structures for some of the common metals: § Body‑centered cubic (BCC) § Chromium, Iron, Molybdenum, Tungsten § Face‑centered cubic (FCC) § Aluminum, Copper, Gold, Lead, Silver, Nickel § Hexagonal close‑packed (HCP) § Magnesium, Titanium, Zinc © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

Imperfections (Defects) in Crystals § Imperfections often arise due to inability of solidifying material to continue replication of unit cell, e. g. , grain boundaries in metals § Imperfections can also be introduced purposely; e. g. , addition of alloying ingredient in metal § Types of defects: 1. Point defects 2. Line defects 3. Surface defects © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

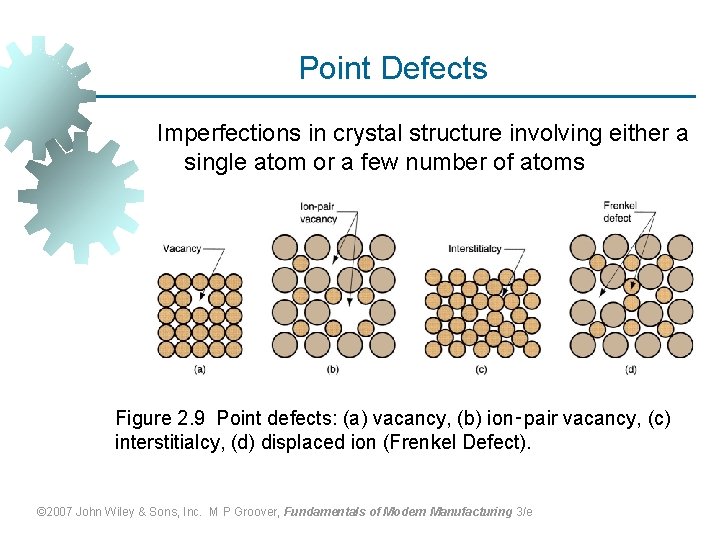
Point Defects Imperfections in crystal structure involving either a single atom or a few number of atoms Figure 2. 9 Point defects: (a) vacancy, (b) ion‑pair vacancy, (c) interstitialcy, (d) displaced ion (Frenkel Defect). © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

Line Defects Connected group of point defects that forms a line in the lattice structure § Most important line defect is a dislocation, which can take two forms: § Edge dislocation § Screw dislocation © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

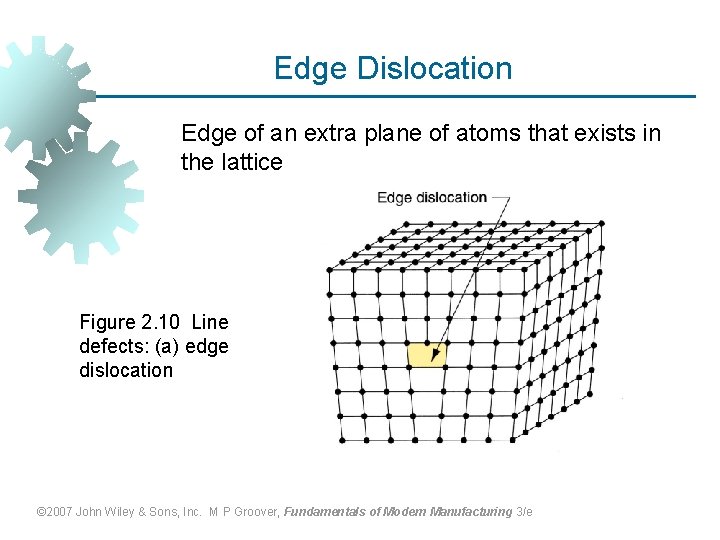
Edge Dislocation Edge of an extra plane of atoms that exists in the lattice Figure 2. 10 Line defects: (a) edge dislocation © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

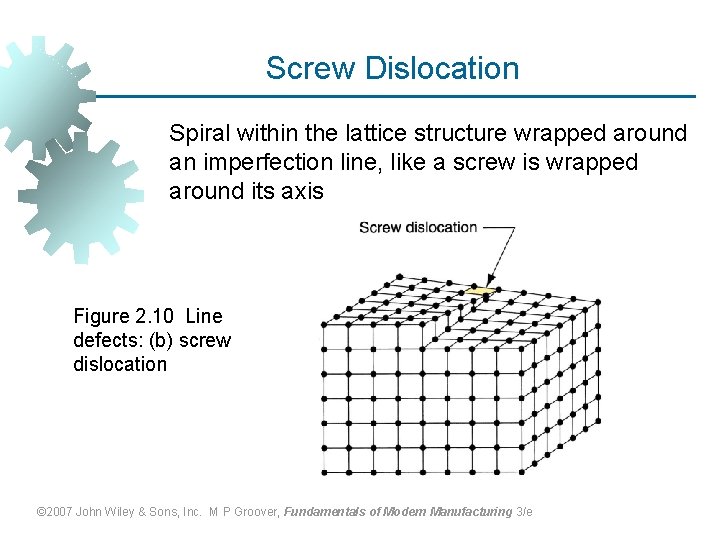
Screw Dislocation Spiral within the lattice structure wrapped around an imperfection line, like a screw is wrapped around its axis Figure 2. 10 Line defects: (b) screw dislocation © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

Surface Defects Imperfections that extend in two directions to form a boundary § Examples: § External: the surface of a crystalline object is an interruption in the lattice structure § Internal: grain boundaries are internal surface interruptions © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

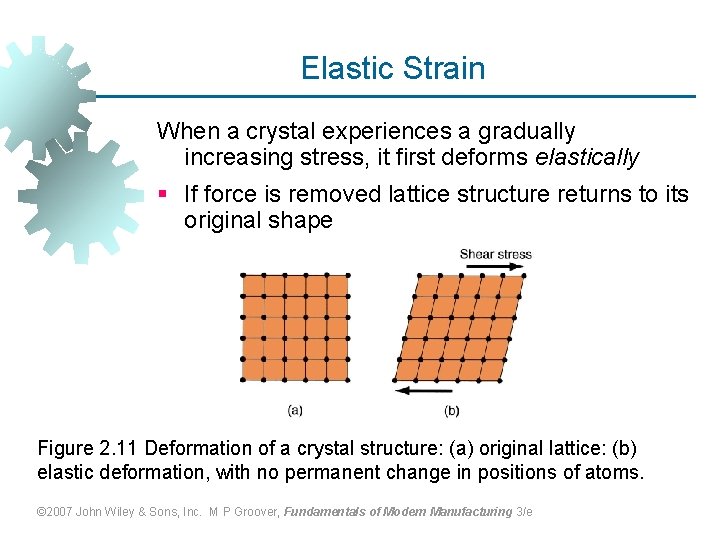
Elastic Strain When a crystal experiences a gradually increasing stress, it first deforms elastically § If force is removed lattice structure returns to its original shape Figure 2. 11 Deformation of a crystal structure: (a) original lattice: (b) elastic deformation, with no permanent change in positions of atoms. © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

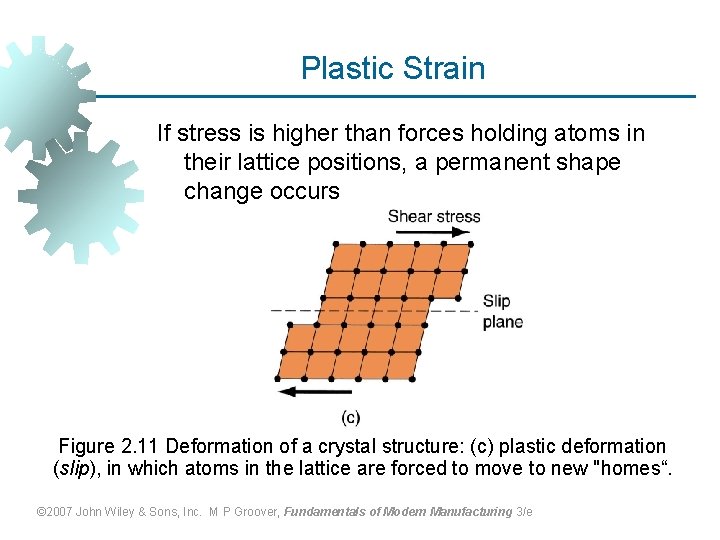
Plastic Strain If stress is higher than forces holding atoms in their lattice positions, a permanent shape change occurs Figure 2. 11 Deformation of a crystal structure: (c) plastic deformation (slip), in which atoms in the lattice are forced to move to new "homes“. © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

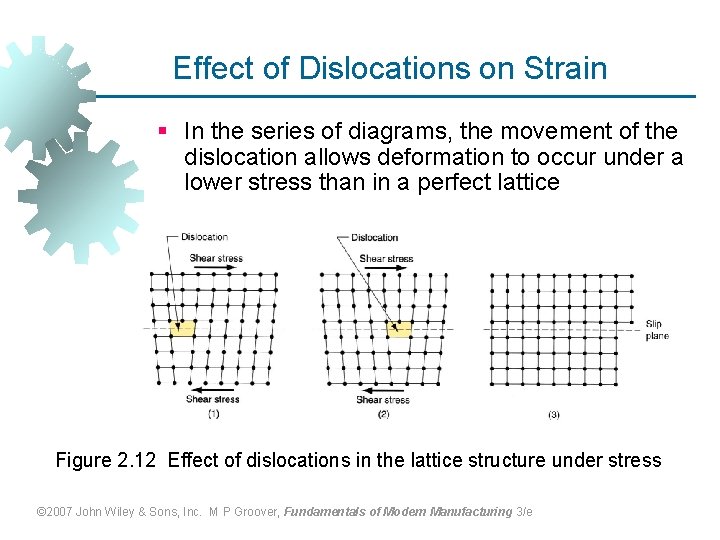
Effect of Dislocations on Strain § In the series of diagrams, the movement of the dislocation allows deformation to occur under a lower stress than in a perfect lattice Figure 2. 12 Effect of dislocations in the lattice structure under stress © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

Slip on a Macroscopic Scale § Slip occurs many times over throughout the metal when subjected to a deforming load, thus causing it to exhibit its macroscopic behavior in the stress-strain relationship § Dislocations are a good‑news‑bad‑news situation § Good news in manufacturing – the metal is easier to form § Bad news in design – the metal is not as strong as the designer would like © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

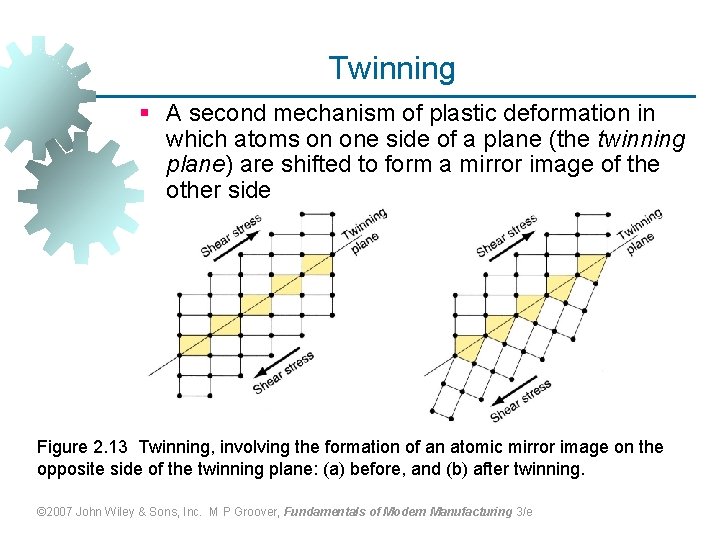
Twinning § A second mechanism of plastic deformation in which atoms on one side of a plane (the twinning plane) are shifted to form a mirror image of the other side Figure 2. 13 Twinning, involving the formation of an atomic mirror image on the opposite side of the twinning plane: (a) before, and (b) after twinning. © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

Polycrystalline Nature of Metals § A block of metal may contain millions of individual crystals, called grains § Such a structure is called polycrystalline § Each grain has its own unique lattice orientation; but collectively, the grains are randomly oriented in the block © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

Grains and Grain Boundaries in Metals § How do polycrystalline structures form? § As a block of metal cools from the molten state and begins to solidify, individual crystals nucleate at random positions and orientations throughout the liquid § These crystals grow and finally interfere with each other, forming at their interface a surface defect ‑ a grain boundary § Grain boundaries are transition zones, perhaps only a few atoms thick © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e

Thanks © 2007 John Wiley & Sons, Inc. M P Groover, Fundamentals of Modern Manufacturing 3/e