The Myofilament Ca 2 Sensitizer Levosimendan Preserves Systolic







- Slides: 19

The Myofilament Ca 2+ Sensitizer Levosimendan Preserves Systolic Function in Rats with Volume Overload Heart Failure Kristin Lewis, DVM Pathology Resident/Graduate Research Associate The Ohio State University, Columbus, OH The Research Institute, Nationwide Children’s Hospital, Columbus, OH

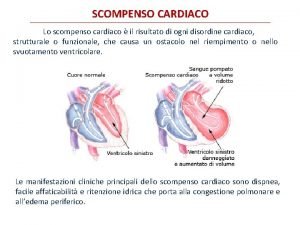
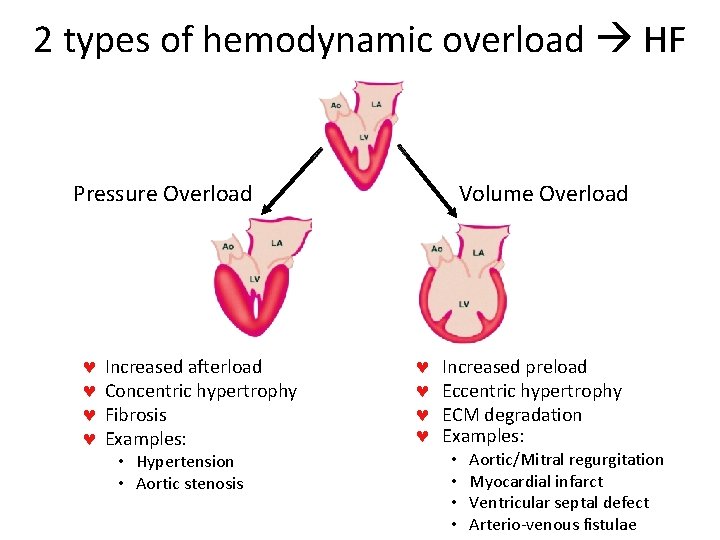
2 types of hemodynamic overload HF Volume Overload Pressure Overload © © Increased afterload Concentric hypertrophy Fibrosis Examples: • Hypertension • Aortic stenosis © © Increased preload Eccentric hypertrophy ECM degradation Examples: • • Aortic/Mitral regurgitation Myocardial infarct Ventricular septal defect Arterio-venous fistulae

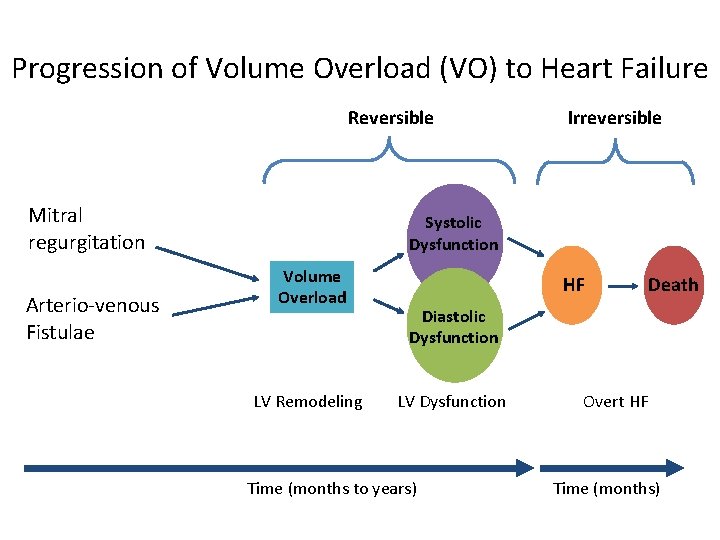
Progression of Volume Overload (VO) to Heart Failure Reversible Mitral regurgitation Arterio-venous Fistulae Irreversible Systolic Dysfunction Volume Overload LV Remodeling HF Death Diastolic Dysfunction LV Dysfunction Time (months to years) Overt HF Time (months)

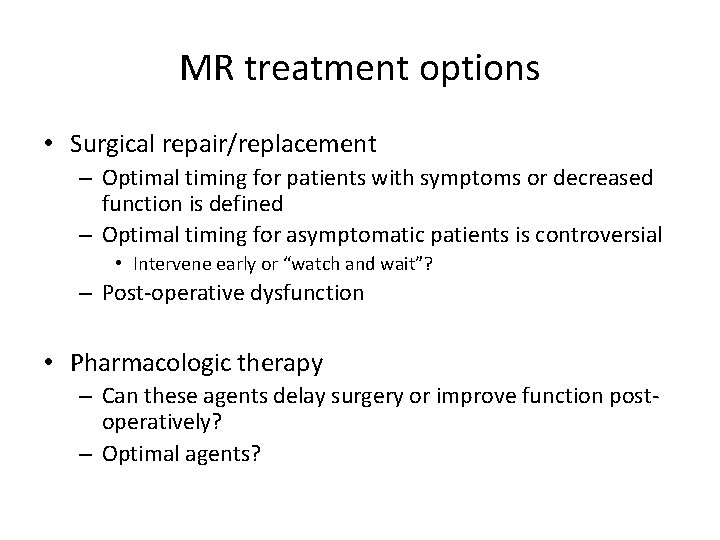
MR treatment options • Surgical repair/replacement – Optimal timing for patients with symptoms or decreased function is defined – Optimal timing for asymptomatic patients is controversial • Intervene early or “watch and wait”? – Post-operative dysfunction • Pharmacologic therapy – Can these agents delay surgery or improve function postoperatively? – Optimal agents?

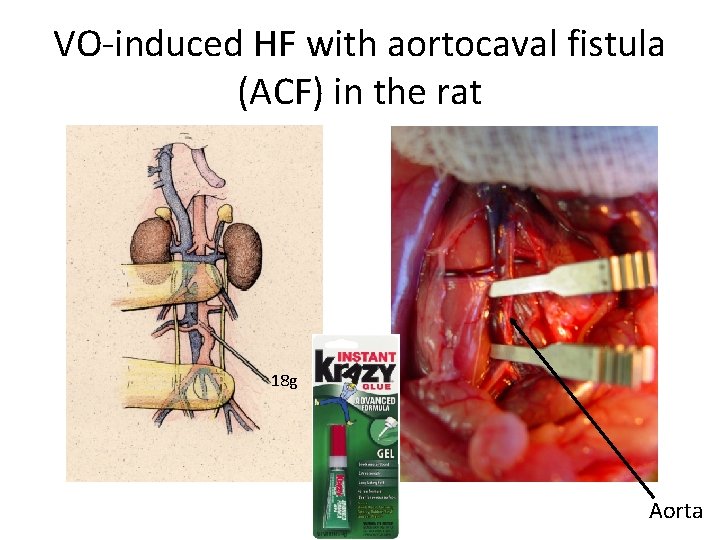
VO-induced HF with aortocaval fistula (ACF) in the rat 18 g Aorta

ACF progressive increase in LVEDd, LVEDs Chest wall “Anterior” Sham LVEDd LVEDs 4 wk ACF “Posterior” Time 8 wk ACF 15 wk ACF

VO is accompanied by functional deterioration % Fractional Shortening * * LVEDd LVEDs *= P < 0. 05 vs. Sham

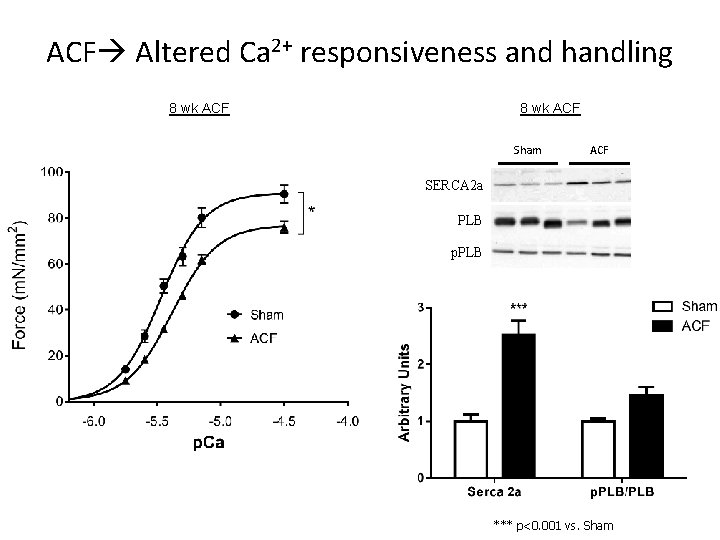
ACF Altered Ca 2+ responsiveness and handling 8 wk ACF Sham ACF SERCA 2 a PLB p. PLB *** p<0. 001 vs. Sham

Hypothesis Therapeutic strategies targeting myofilament Ca 2+ sensitivity will preserve/improve LV function in valvular heart disease

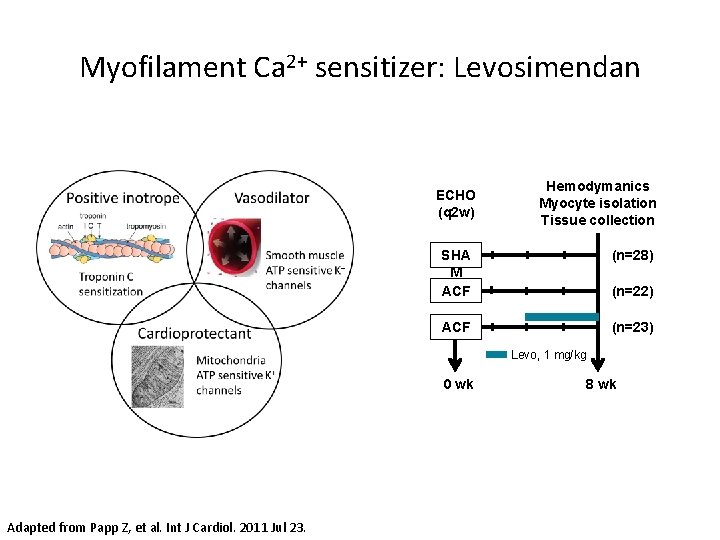
Myofilament Ca 2+ sensitizer: Levosimendan ECHO (q 2 w) Hemodymanics Myocyte isolation Tissue collection SHA M ACF (n=22) ACF (n=23) (n=28) Levo, 1 mg/kg 0 wk Adapted from Papp Z, et al. Int J Cardiol. 2011 Jul 23. 8 wk

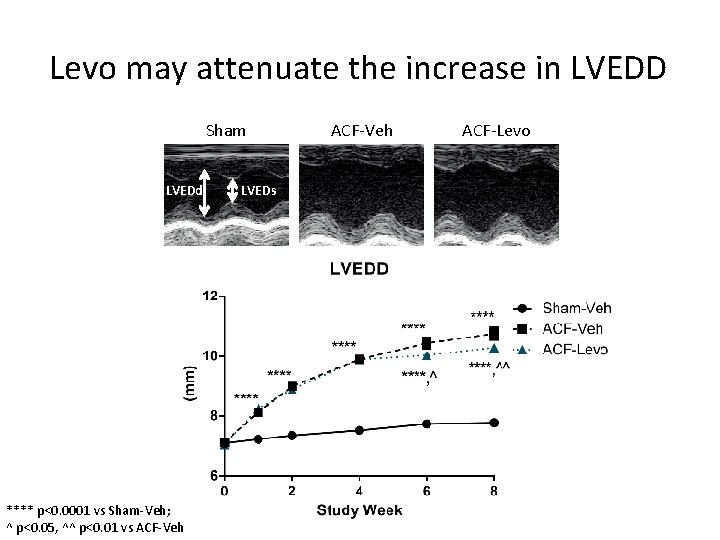
Levo may attenuate the increase in LVEDD Sham LVEDd **** p<0. 0001 vs Sham-Veh; ^ p<0. 05, ^^ p<0. 01 vs ACF-Veh LVEDs ACF-Veh ACF-Levo

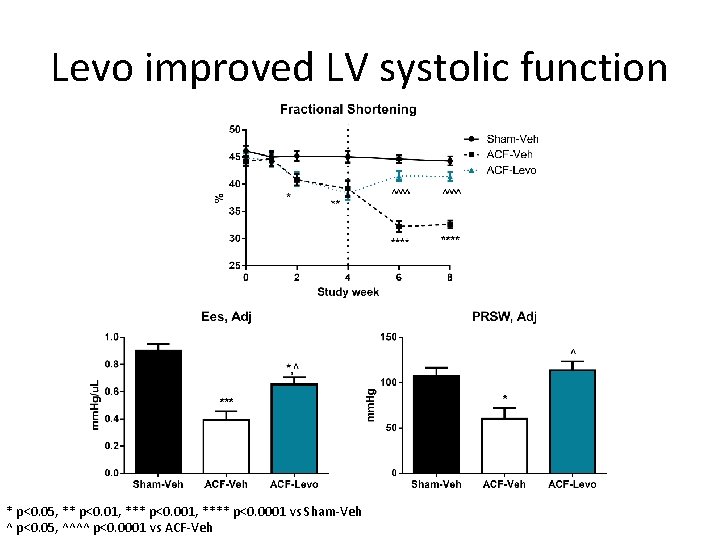
Levo improved LV systolic function * p<0. 05, ** p<0. 01, *** p<0. 001, **** p<0. 0001 vs Sham-Veh ^ p<0. 05, ^^^^ p<0. 0001 vs ACF-Veh

Levo ↑myofilament Ca 2+ sensitivity & ↑ maximal force without ↑ Ca 2+ transient * p<0. 05, ** p<0. 01 vs Sham-Veh ^ p<0. 05, ^^^ p<0. 001, ^^^^ p<0. 0001 vs ACF-Veh

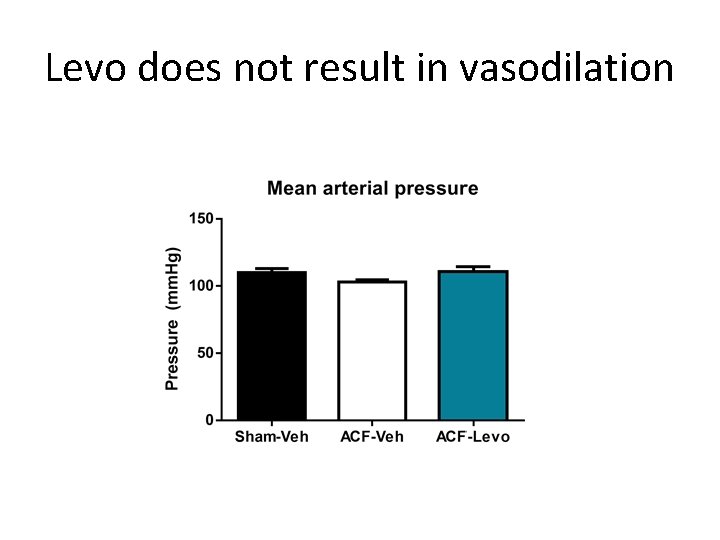
Levo does not result in vasodilation

Levo improved LV diastolic function **** p<0. 0001 vs Sham-Veh ^ p<0. 05, ^^^ p<0. 001 vs ACF-Veh

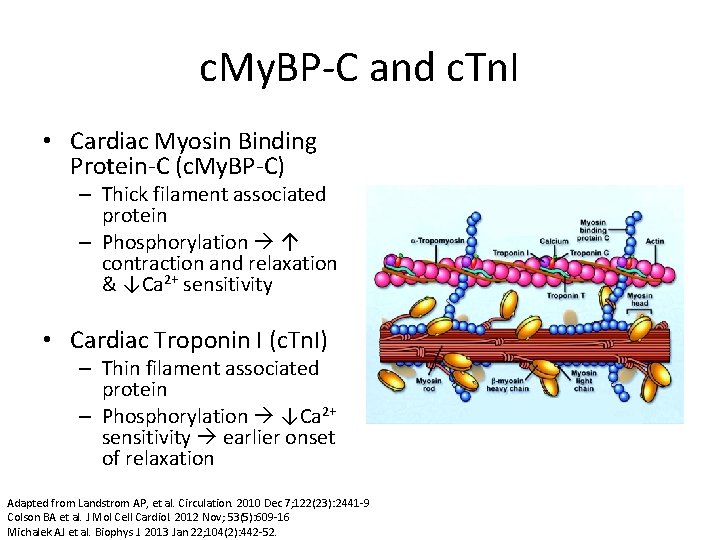
c. My. BP-C and c. Tn. I • Cardiac Myosin Binding Protein-C (c. My. BP-C) – Thick filament associated protein – Phosphorylation ↑ contraction and relaxation & ↓Ca 2+ sensitivity • Cardiac Troponin I (c. Tn. I) – Thin filament associated protein – Phosphorylation ↓Ca 2+ sensitivity earlier onset of relaxation Adapted from Landstrom AP, et al. Circulation. 2010 Dec 7; 122(23): 2441 -9 Colson BA et al. J Mol Cell Cardiol. 2012 Nov; 53(5): 609 -16 Michalek AJ et al. Biophys J. 2013 Jan 22; 104(2): 442 -52.

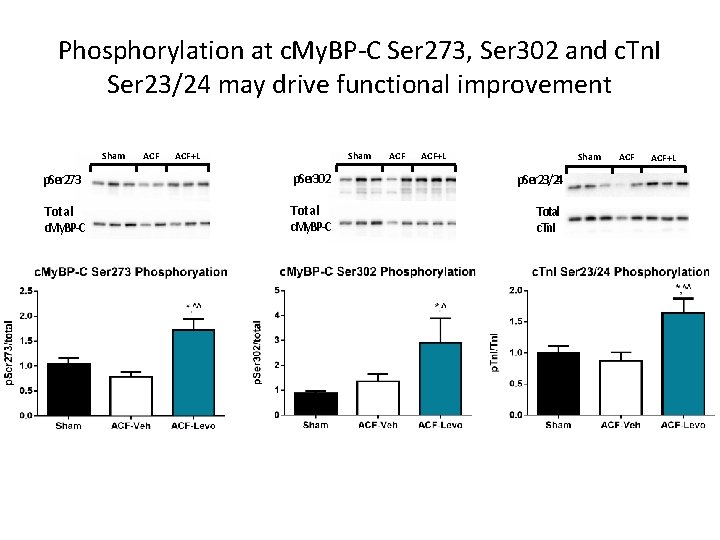
Phosphorylation at c. My. BP-C Ser 273, Ser 302 and c. Tn. I Ser 23/24 may drive functional improvement Sham ACF+L Sham p. Ser 273 p. Ser 302 p. Ser 23/24 Total c. My. BP-C Total c. Tn. I ACF+L

Summary § Myofilament Ca 2+ sensitizer therapy improved systolic and diastolic function § Improved systolic function is due to increased myofilament Ca 2+ sensitivity § Improved diastolic function may be due to c. My. BP-C and/or c. Tn. I phosphorylation § Myofilament Ca 2+ sensitizer therapy mildly attenuated increase in LVEDD § Therapeutic strategies targeting myofilament Ca 2+ sensitivity may improve function prior to load reduction surgery

Acknowledgements Nationwide Children’s Hospital • Lucchesi lab – – – – Pam Lucchesi Aaron Trask Aaron West Jean Zhang Anu Guggilam Kirk Hutchinson Mary Cismowski • Vivarium – Natalie Snyder – Brenna Barbour – Erin Grove The Ohio State University • Veterinary Biosciences Funding Sources • ACVP/STP Coalition Fellowship & Genentech • NIH R 01 -HL 056046 • Nationwide Children’s
 Myofilament
Myofilament Myofilament
Myofilament Myofilament
Myofilament Inotropicos
Inotropicos Medicamentos inotropicos
Medicamentos inotropicos Levosimendan farmacocinetica
Levosimendan farmacocinetica Levosimedan
Levosimedan Isolated systolic hypertension
Isolated systolic hypertension Sam systolic anterior motion
Sam systolic anterior motion Tapping sounds
Tapping sounds Creative commons
Creative commons Auscultatory gap
Auscultatory gap What is systolic and diastolic pressure
What is systolic and diastolic pressure Mitral regurgitation murmur
Mitral regurgitation murmur 174/116 blood pressure
174/116 blood pressure Systolic array
Systolic array Mean stroke volume
Mean stroke volume Machinary murmur
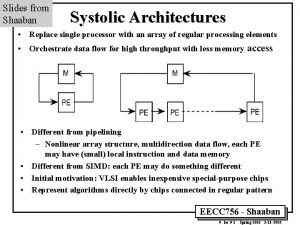
Machinary murmur Why systolic architectures
Why systolic architectures Grading of murmer
Grading of murmer