THE MOLECULES OF LIFE Biosynthesis MOLECULES AND CHEMICAL
THE MOLECULES OF LIFE Biosynthesis
MOLECULES AND CHEMICAL BONDS Molecules two or more atoms of same element covalently bonded Compounds two or more atoms of different elements covalently bonded
ORGANIC MOLECULES Carbon compounds and functional groups carbohydrates lipids proteins nucleic acids For all groups you must be able to identify: • Functions • General Properties of the Molecules • Structure • The Monomer • The Polymer • Examples • The Monomer • The Polymer
MONOMERS AND POLYMERS Monomers subunits of macromolecules DNA has 4 different monomers (nucleotides) proteins have 20 different monomers (amino acids) Polymers series of monomers bonded together Polymerization the bonding of monomers together to form a polymer caused by a reaction called dehydration synthesis
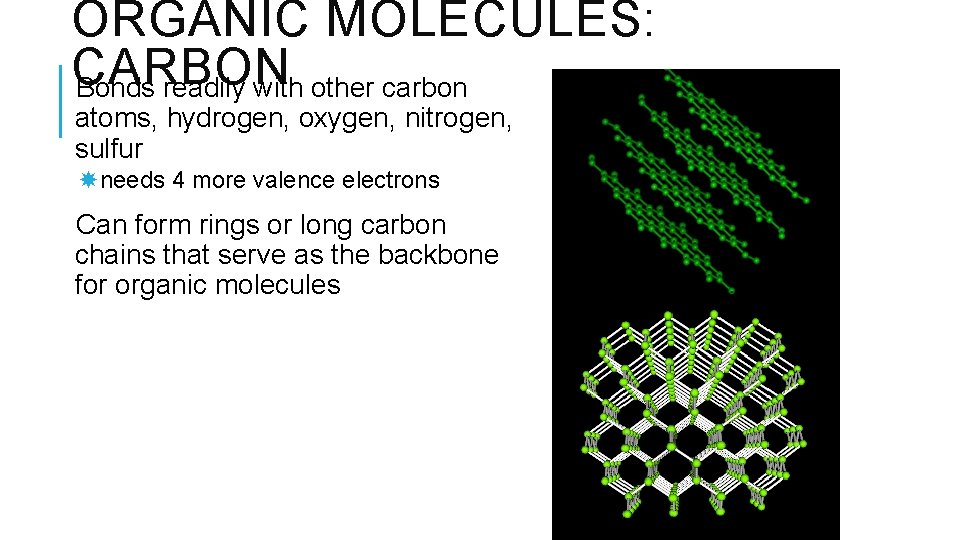
ORGANIC MOLECULES: CARBON Bonds readily with other carbon atoms, hydrogen, oxygen, nitrogen, sulfur needs 4 more valence electrons Can form rings or long carbon chains that serve as the backbone for organic molecules
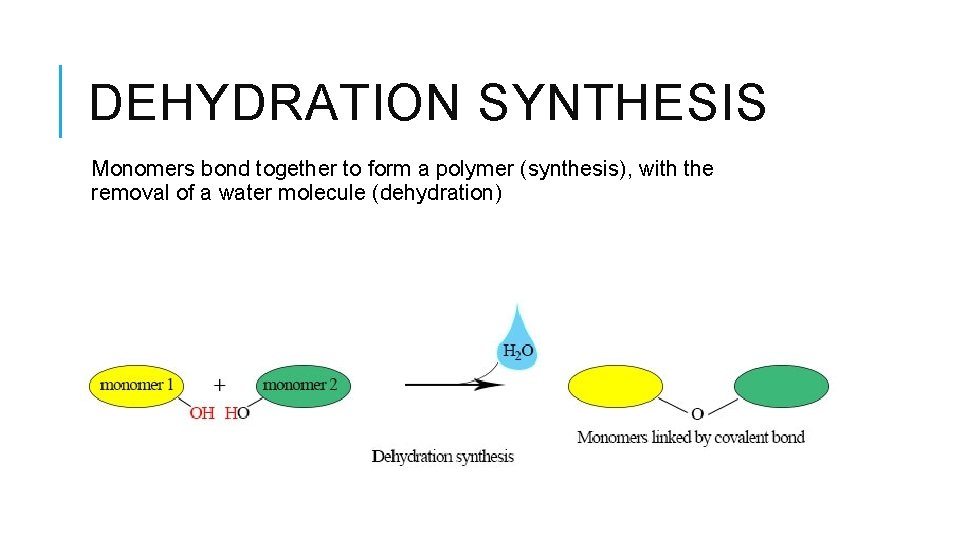
DEHYDRATION SYNTHESIS Monomers bond together to form a polymer (synthesis), with the removal of a water molecule (dehydration)
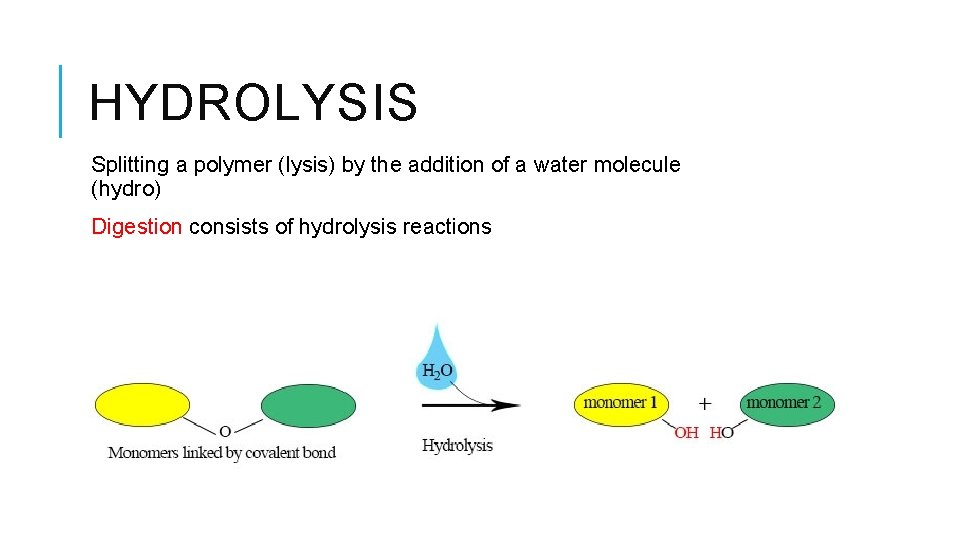
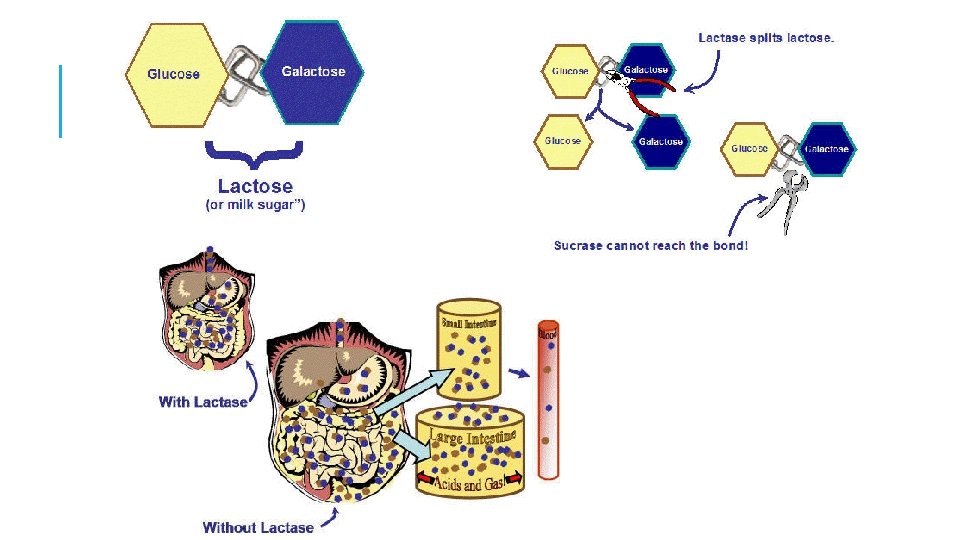
HYDROLYSIS Splitting a polymer (lysis) by the addition of a water molecule (hydro) Digestion consists of hydrolysis reactions
CARBOHYDRATES Function/General Characteristics Hydrophilic organic molecule General formula (CH 2 O)n , n = number of carbon atoms for glucose, n = 6, so formula is C 6 H 12 O 6 Names of carbohydrates word root sacchar- or the suffix ose often used monosaccharide or glucose

CARBOHYDRATES Structure – Monomer MONOSACCHARIDE
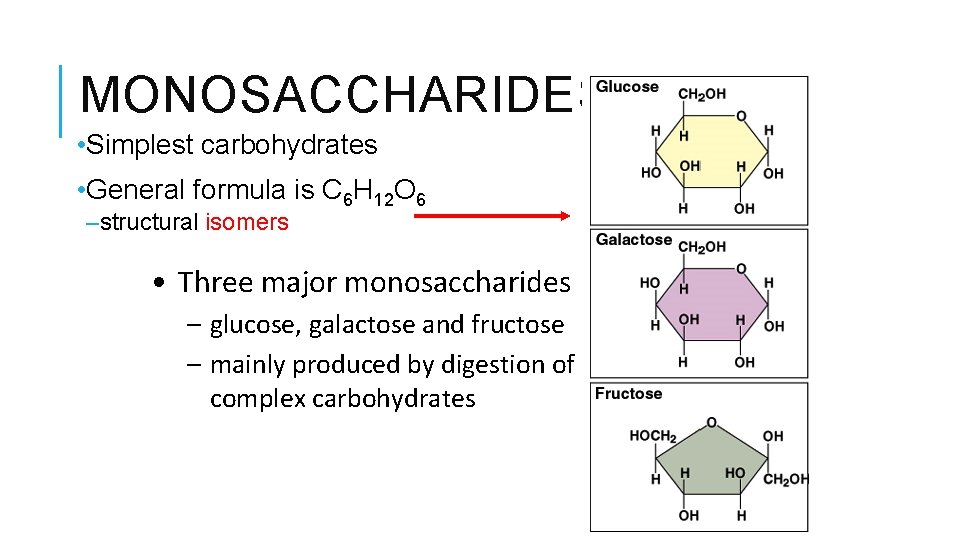
MONOSACCHARIDES • Simplest carbohydrates • General formula is C 6 H 12 O 6 –structural isomers • Three major monosaccharides – glucose, galactose and fructose – mainly produced by digestion of complex carbohydrates
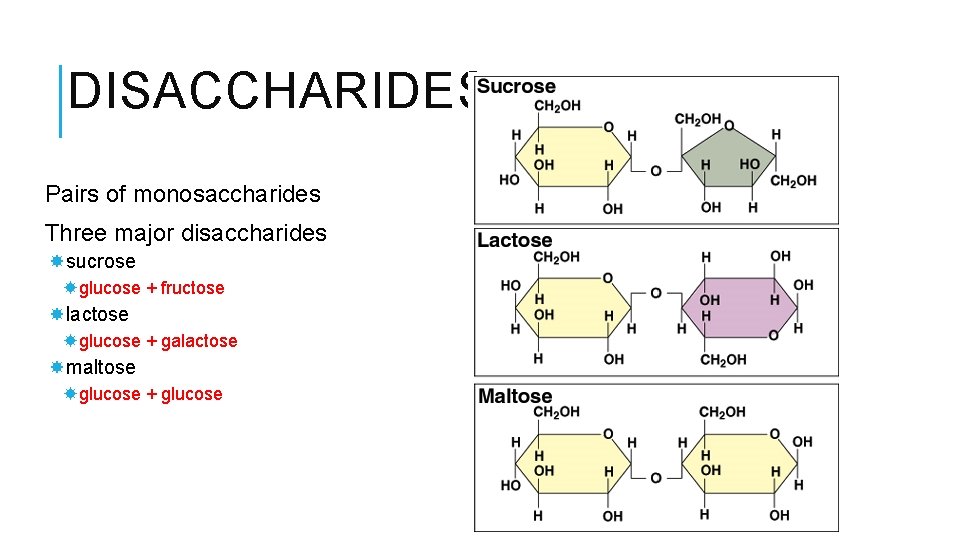
DISACCHARIDES Pairs of monosaccharides Three major disaccharides sucrose glucose + fructose lactose glucose + galactose maltose glucose + glucose

COMMON NAMES Monosaccharides & Disaccharides are commonly called “Simple Carbohydrates” or “Sugars”

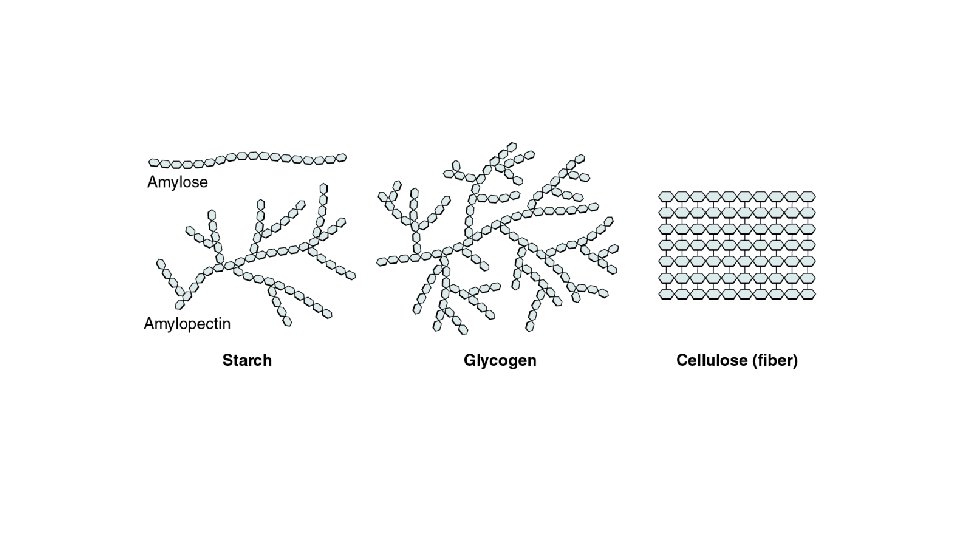
CARBOHYDRATES Structure – Polymer POLYSACCHARIDE
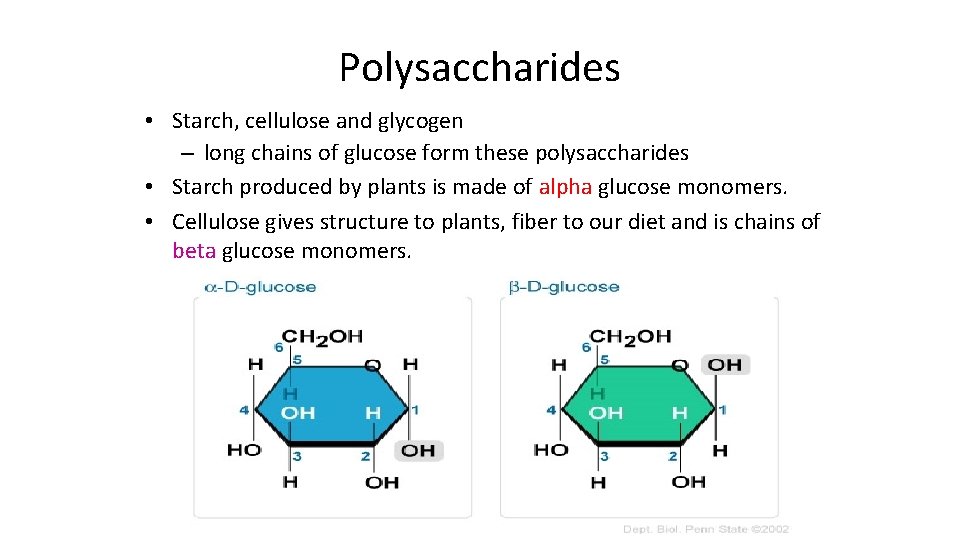
Polysaccharides • Starch, cellulose and glycogen – long chains of glucose form these polysaccharides • Starch produced by plants is made of alpha glucose monomers. • Cellulose gives structure to plants, fiber to our diet and is chains of beta glucose monomers.

COMMON NAMES Polysaccharides are commonly called “Complex Carbohydrates” “Fiber” is the general term for a polysaccharide that is not digestible to us (cellulose).


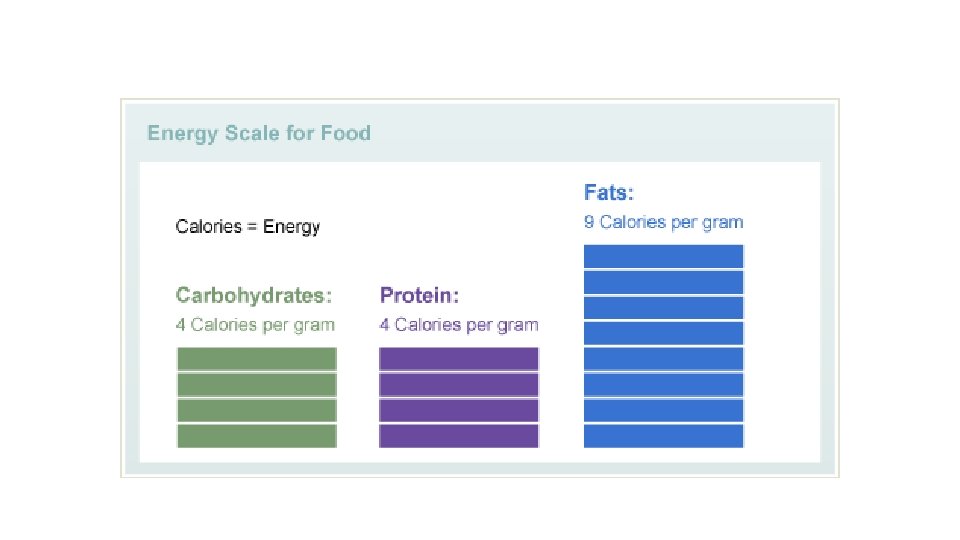
CARBOHYDRATES Function Source of energy !! (roughly 4 calories/gram) Structural Components - Carbohydrates bound to other molecules glycolipids & glycoproteins surface of cell membrane (cell flag) external surface of cell membrane mucus of respiratory and digestive tracts cell adhesion, gelatinous filler of tissues (eye) and lubricates joints

Carbohydrates Video

LIPIDS Function/General Characteristics Hydrophobic organic molecule (bc it’s nonpolar!) Composed of molecules based on chains of carbon

LIPIDS Structure – Monomer FATTY ACID
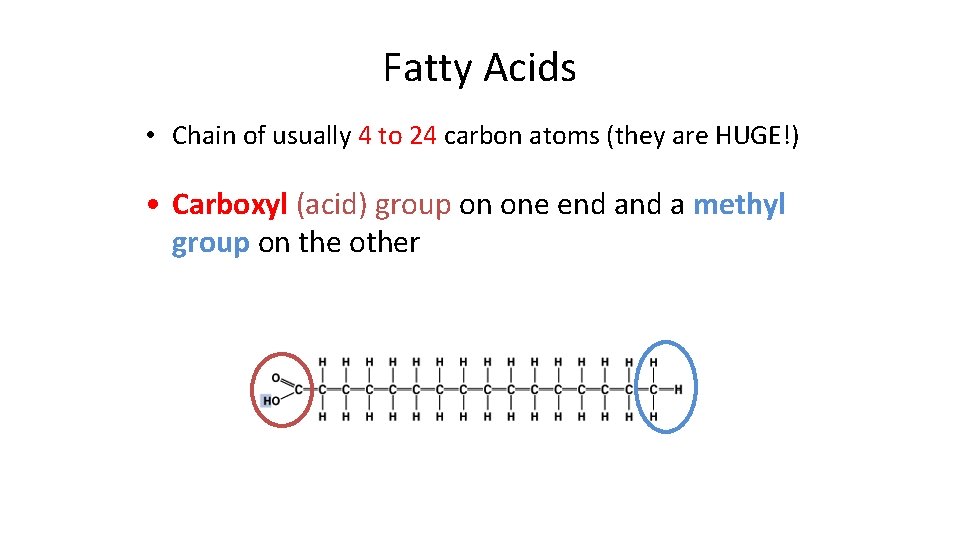
Fatty Acids • Chain of usually 4 to 24 carbon atoms (they are HUGE!) • Carboxyl (acid) group on one end a methyl group on the other
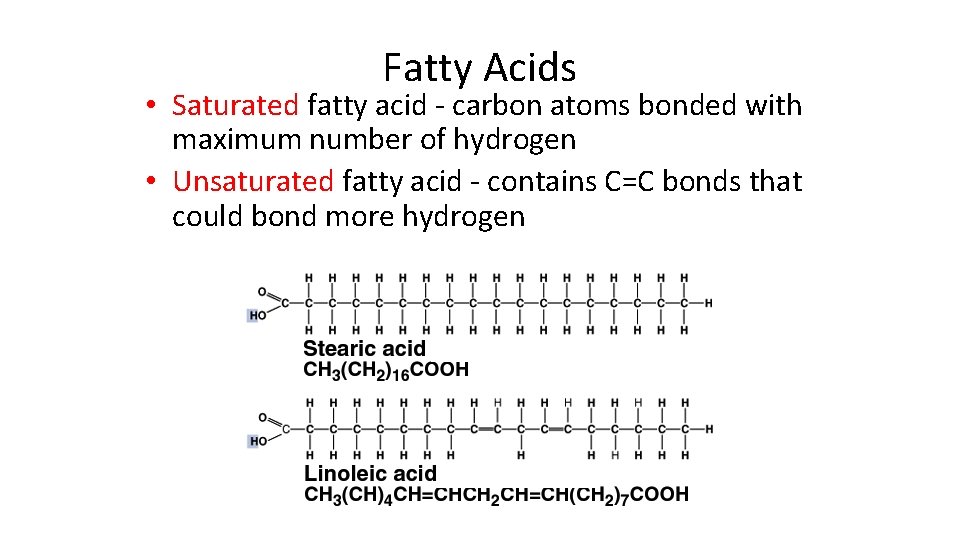
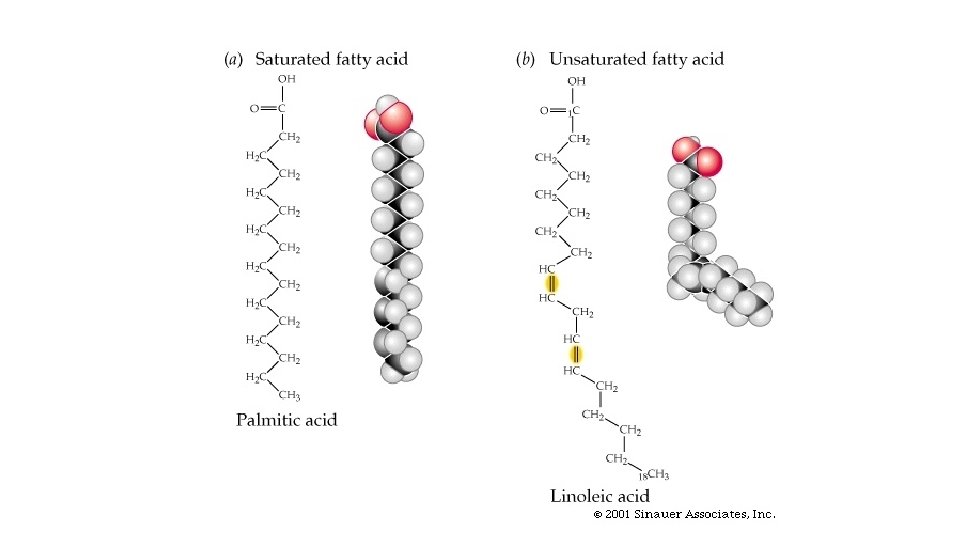
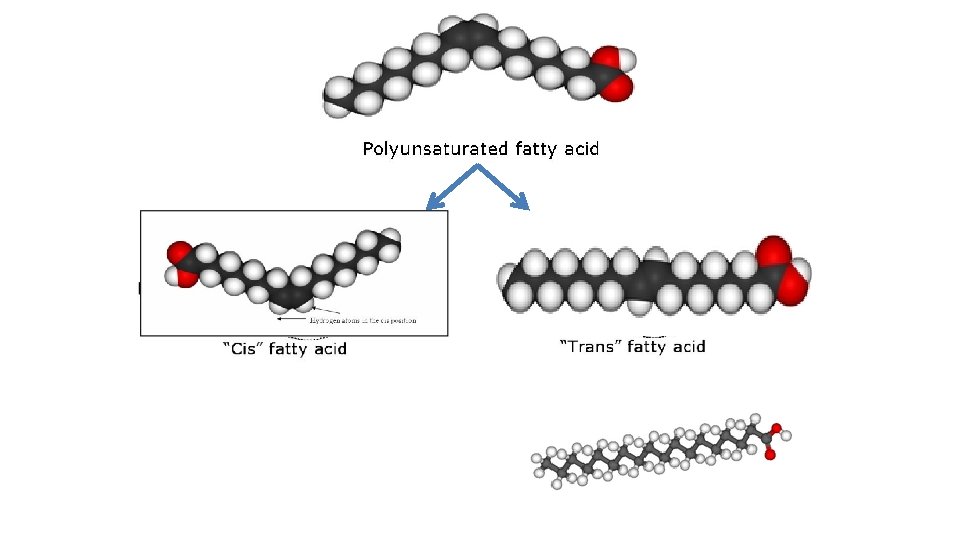
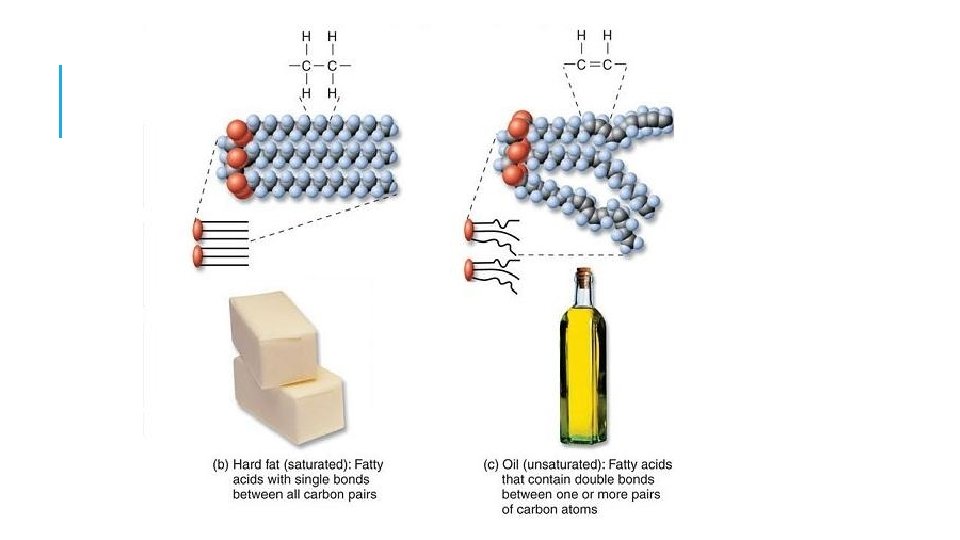
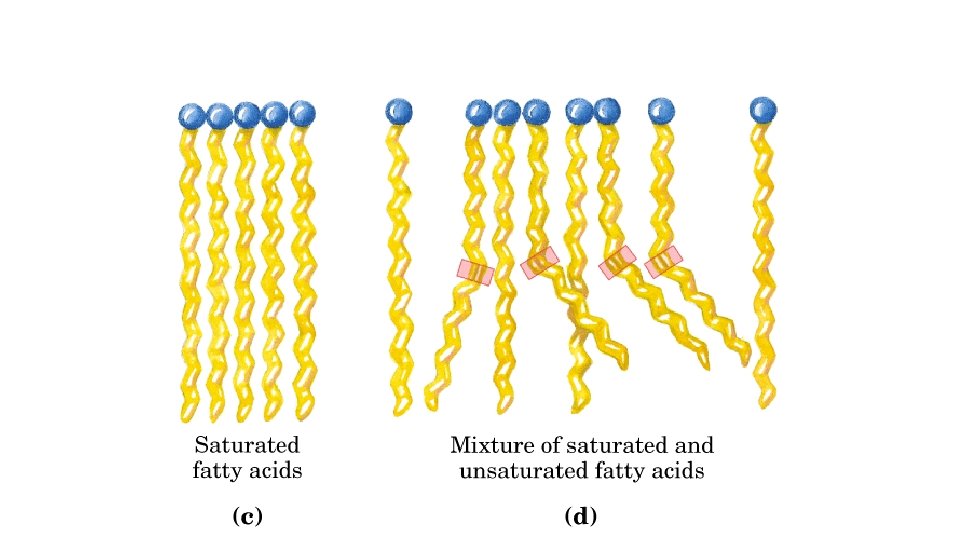
Fatty Acids • Saturated fatty acid - carbon atoms bonded with maximum number of hydrogen • Unsaturated fatty acid - contains C=C bonds that could bond more hydrogen

LIPIDS Structure – Polymer TRIGLYCERIDE PHOSPHOLIPID STERIODS
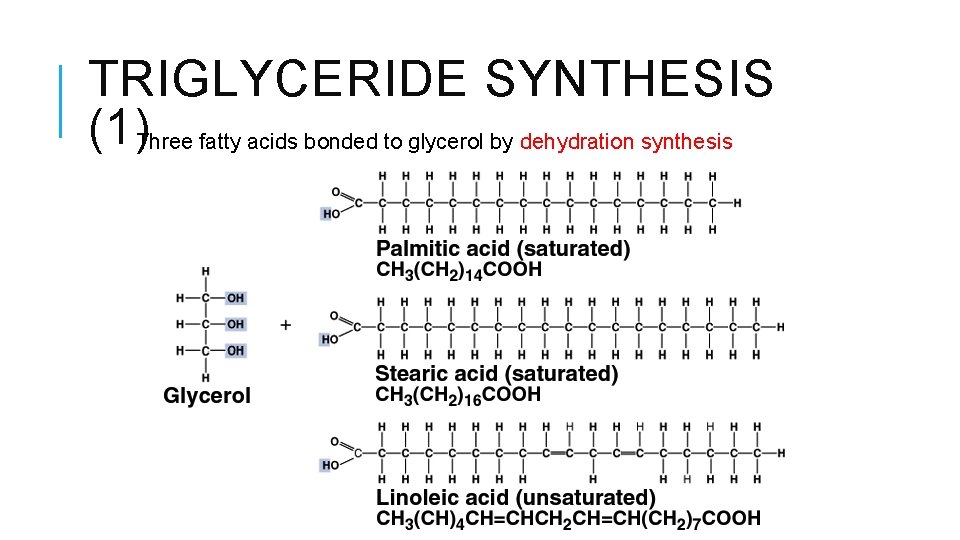
TRIGLYCERIDE SYNTHESIS (1)Three fatty acids bonded to glycerol by dehydration synthesis
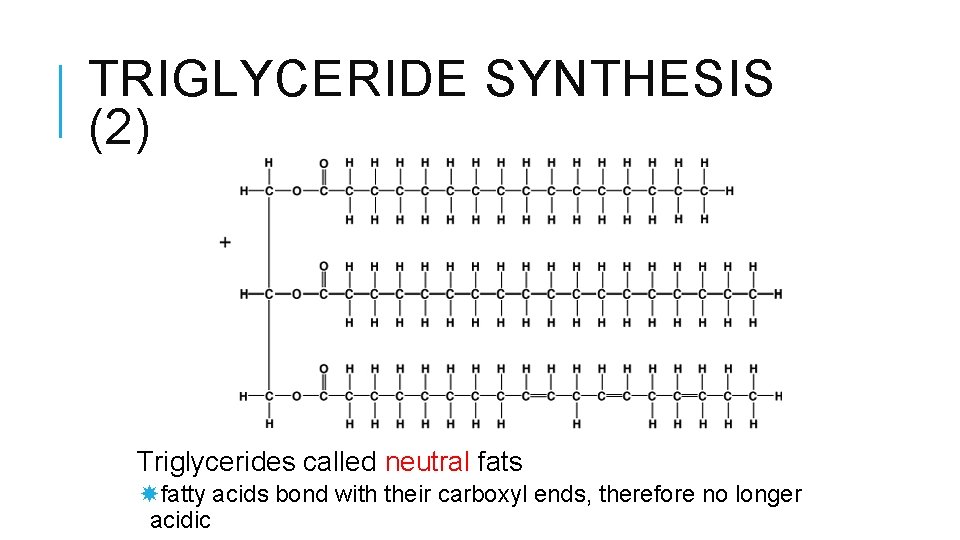
TRIGLYCERIDE SYNTHESIS (2) Triglycerides called neutral fats fatty acids bond with their carboxyl ends, therefore no longer acidic
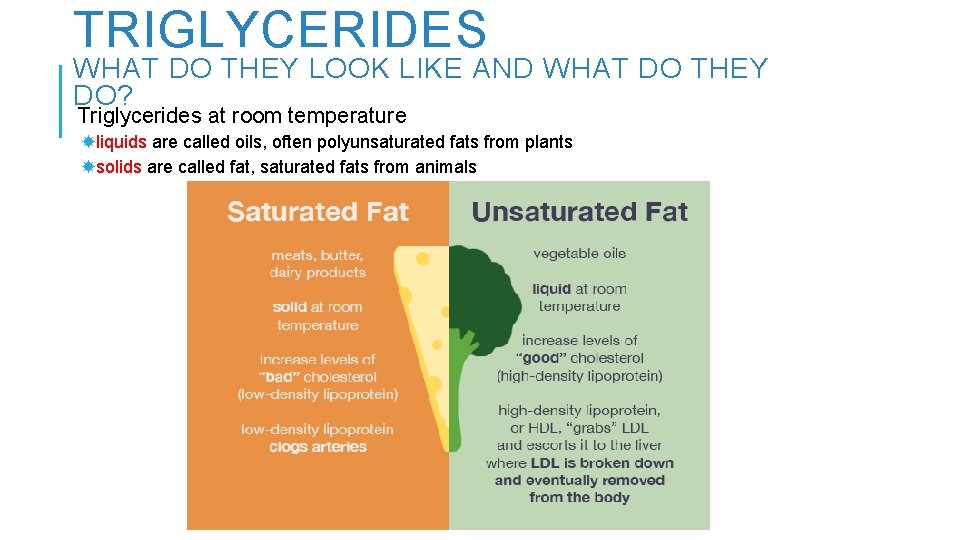
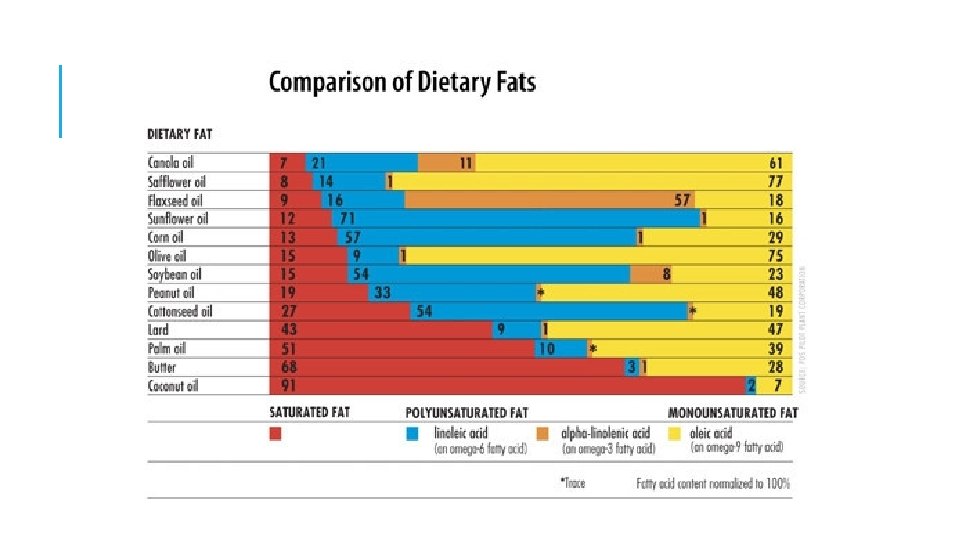
TRIGLYCERIDES WHAT DO THEY LOOK LIKE AND WHAT DO THEY DO? Triglycerides at room temperature liquids are called oils, often polyunsaturated fats from plants solids are called fat, saturated fats from animals

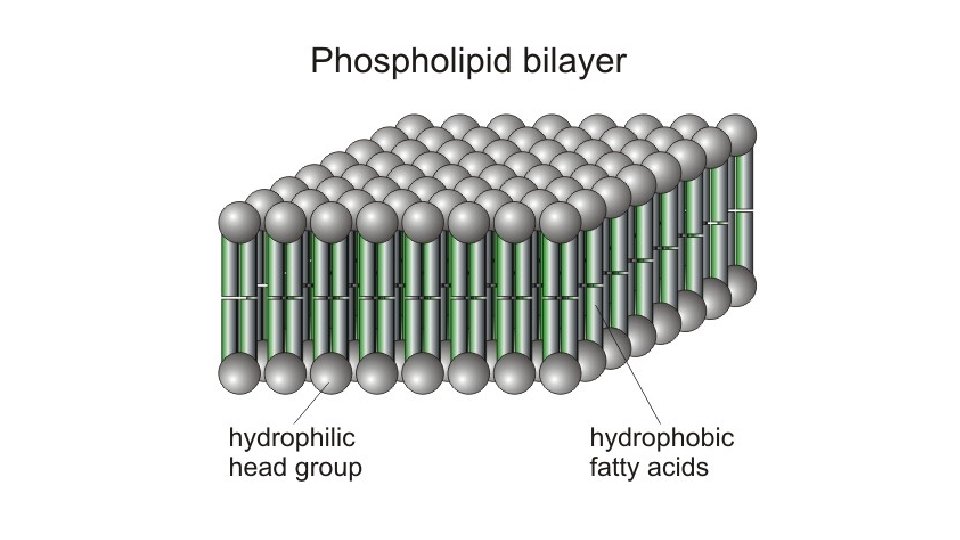
PHOSPHOLIPIDS A VERY CONFUSED MACROMOLECULE! Hydrophobic “tails” similar to neutral fats with two fatty acids attached to glycerol Hydrophilic “head” differs from neutral fat with the one of the fatty acids replaced by a phosphate group attached to other functional groups So does it love or hate water? !? BOTH!
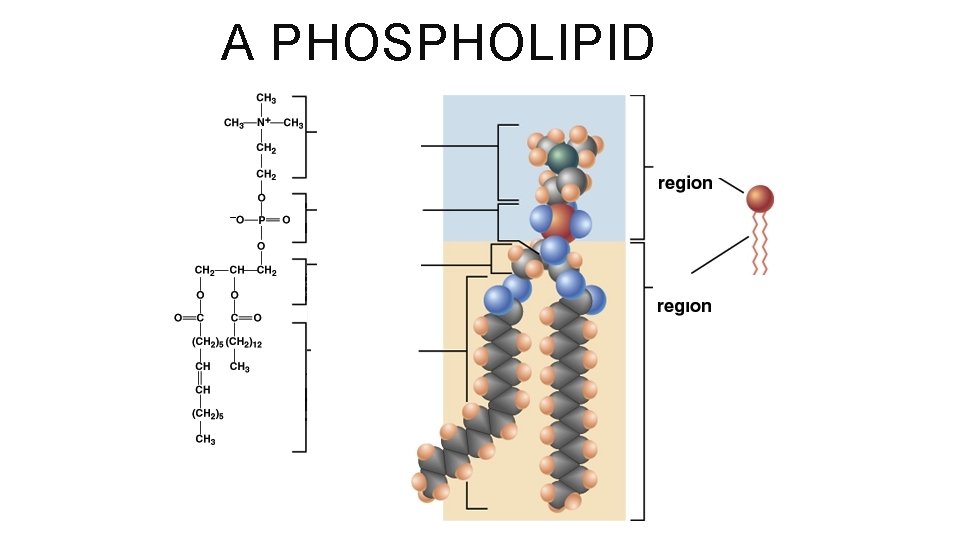
A PHOSPHOLIPID

STEROIDS Cholesterol other steroids derive from cholesterol progesterone, estrogens, testosterone and bile acids required for proper nervous system function and is an important component of cell membranes produced only by animals 85% naturally produced by our body only 15% derived from our diet
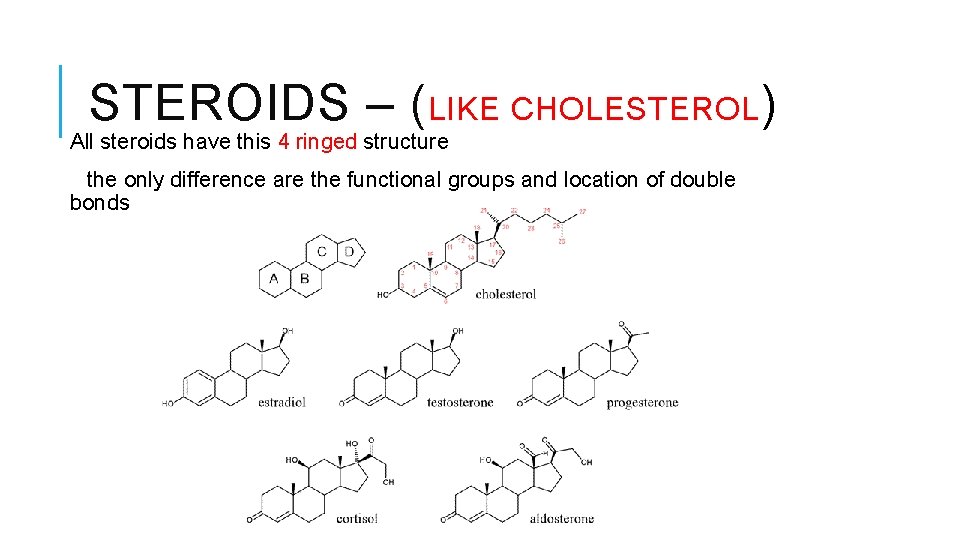
STEROIDS – (LIKE CHOLESTEROL) All steroids have this 4 ringed structure the only difference are the functional groups and location of double bonds

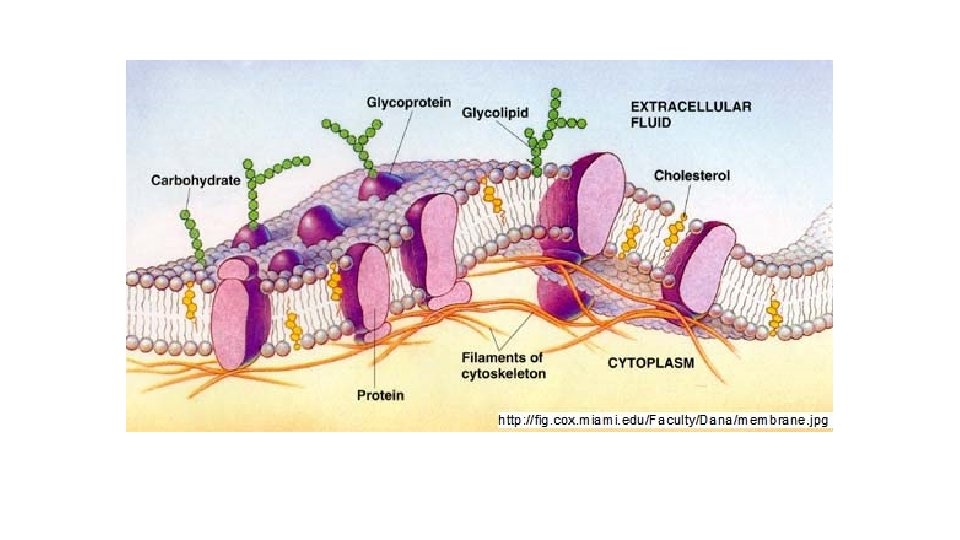
LIPIDS Function Energy Storage (about 9 calories/gram) Structural Components (Cell Membranes) Chemical Messengers (Hormones) Insulation/Absorption

LIPIDS VIDEO

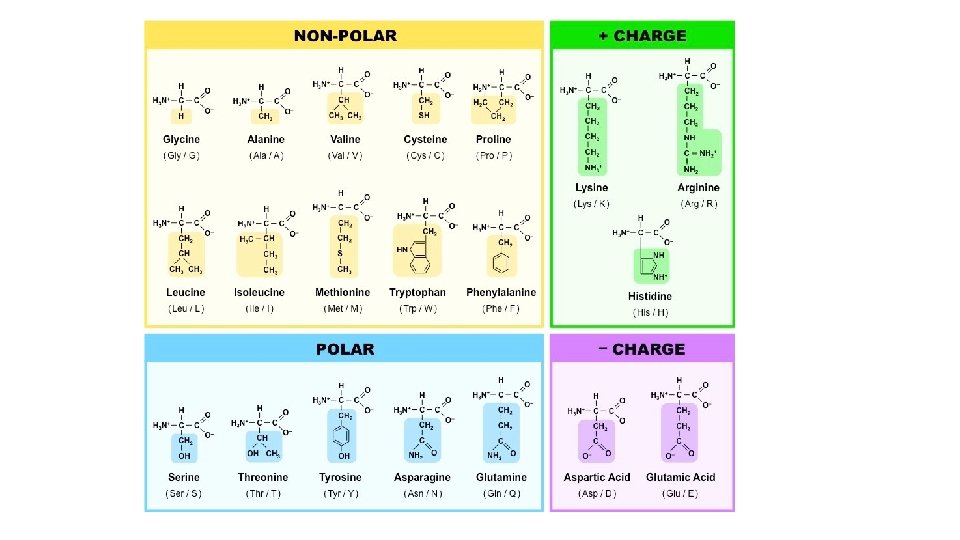
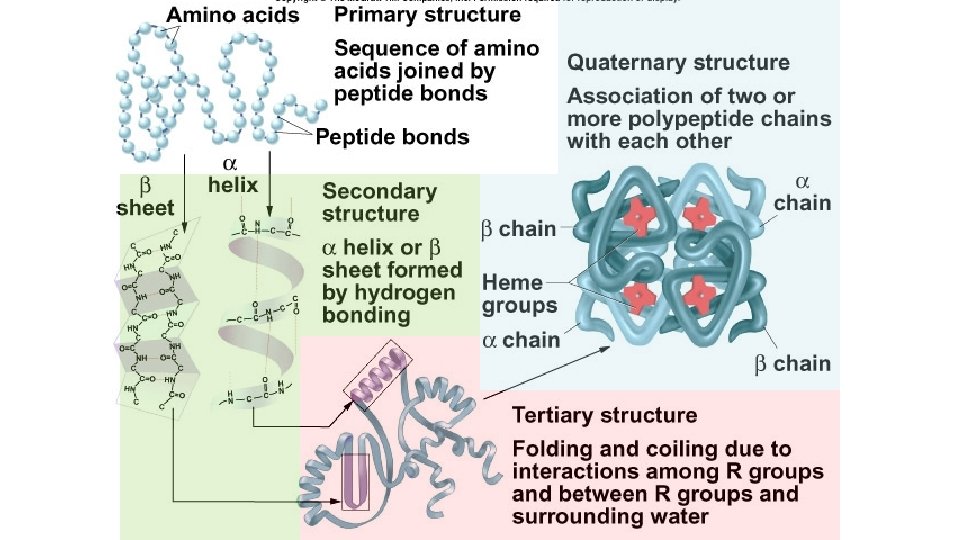
PROTEIN General Properties • Polymer of amino acids • 20 amino acid monomers • The amino acids in a protein determine its structure and function

PROTEIN Monomer AMINO ACID
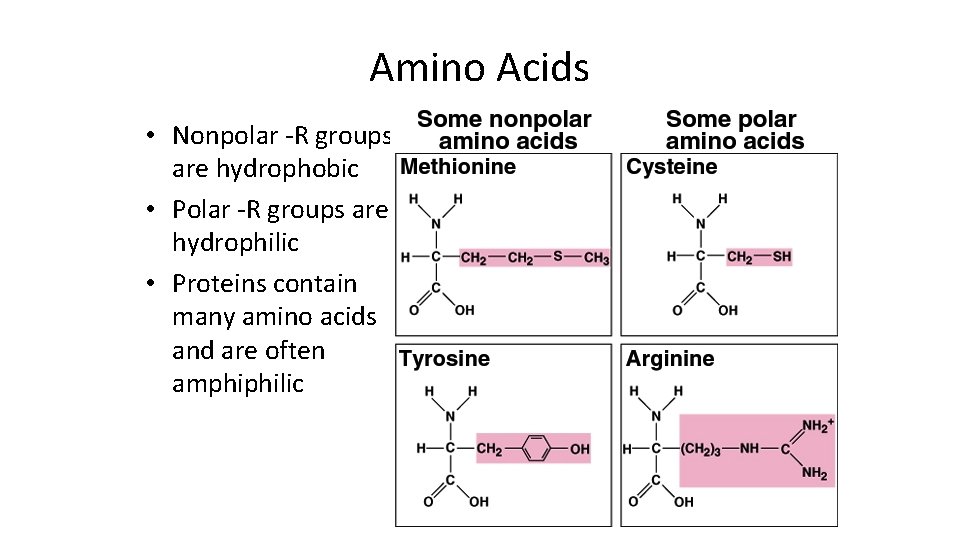
Amino Acids • Nonpolar -R groups are hydrophobic • Polar -R groups are hydrophilic • Proteins contain many amino acids and are often amphiphilic
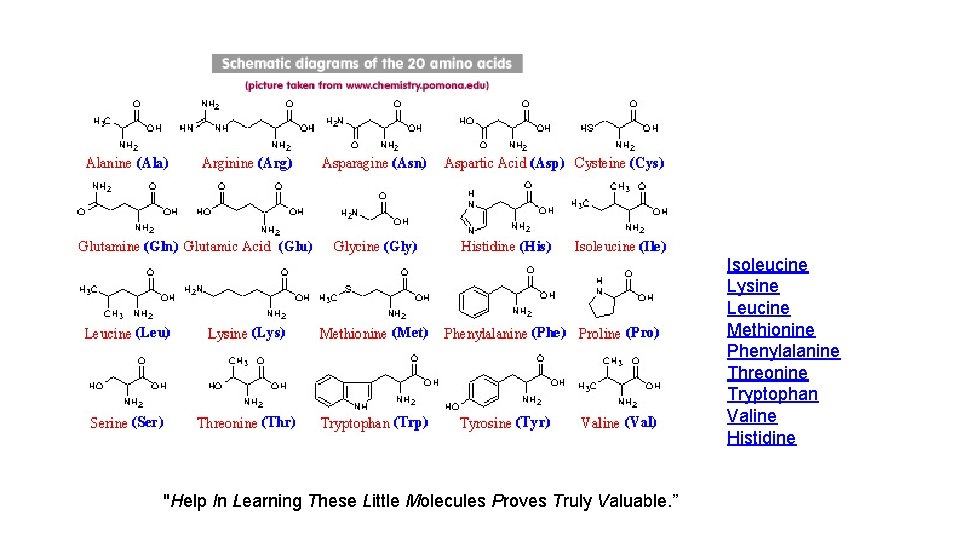
Isoleucine Lysine Leucine Methionine Phenylalanine Threonine Tryptophan Valine Histidine "Help In Learning These Little Molecules Proves Truly Valuable. ”
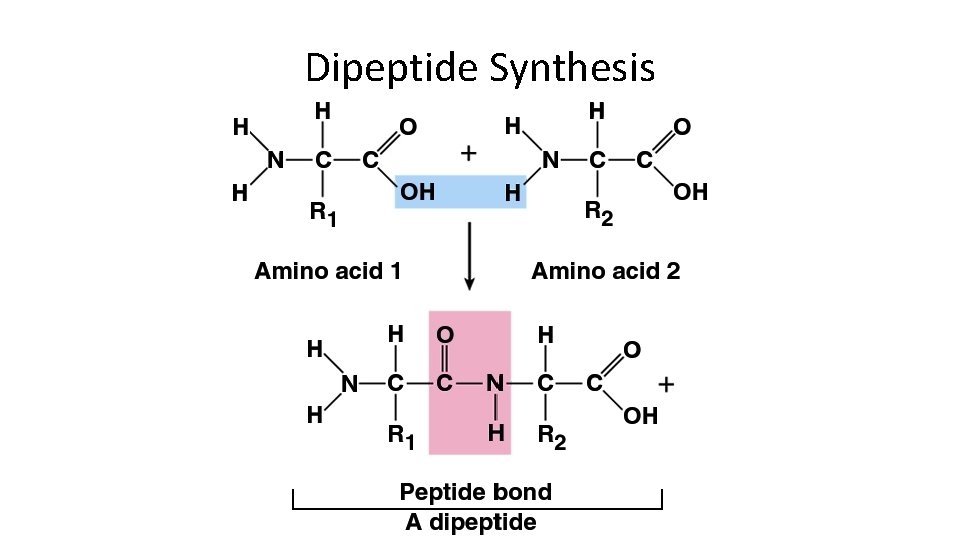
Dipeptide Synthesis

PROTEIN Polymer POLYPEPTIDE or PROTEIN
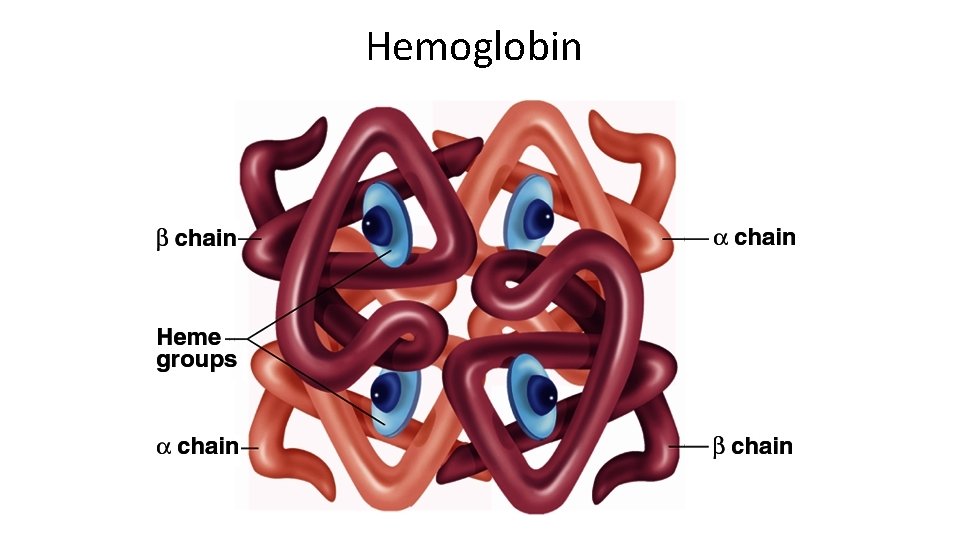
Hemoglobin
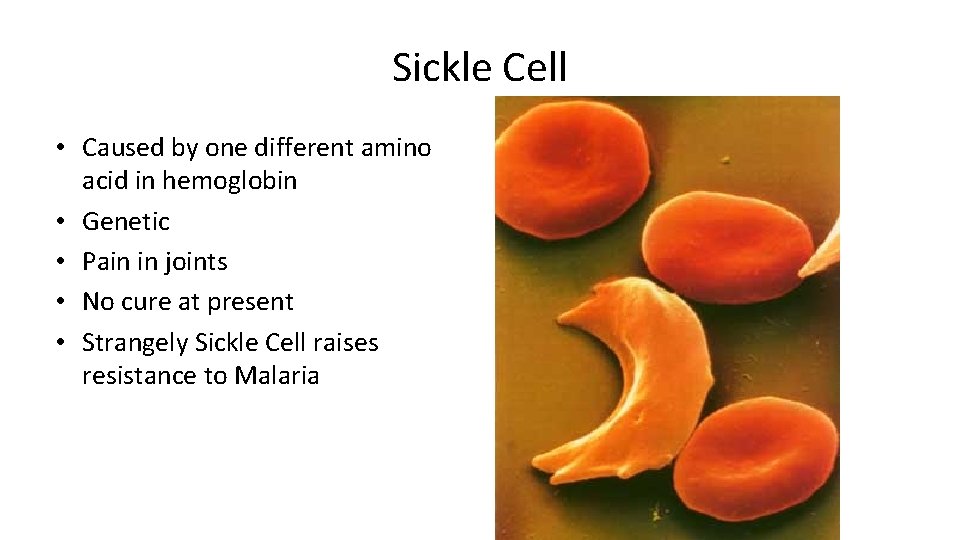
Sickle Cell • Caused by one different amino acid in hemoglobin • Genetic • Pain in joints • No cure at present • Strangely Sickle Cell raises resistance to Malaria
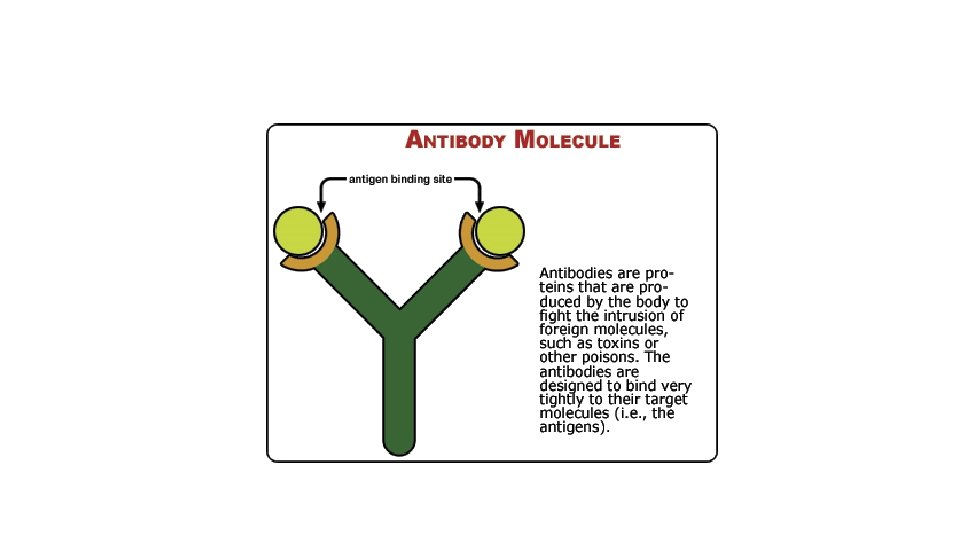
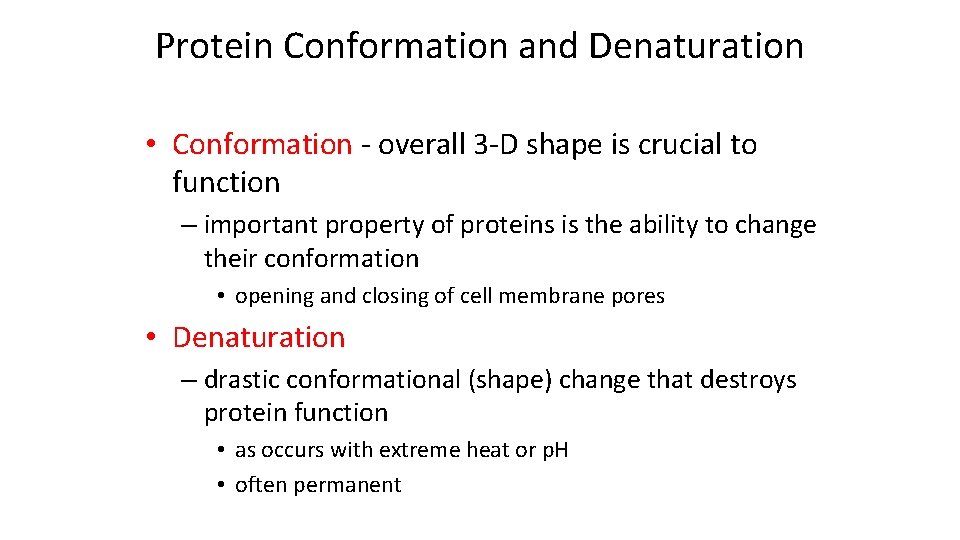
Protein Conformation and Denaturation • Conformation - overall 3 -D shape is crucial to function – important property of proteins is the ability to change their conformation • opening and closing of cell membrane pores • Denaturation – drastic conformational (shape) change that destroys protein function • as occurs with extreme heat or p. H • often permanent
Enzymes • Function as catalysts – promote rapid reaction rates • Lower activation energy – energy needed to get reaction started is lowered • enzymes facilitate molecular interaction

Enzyme Video Clip
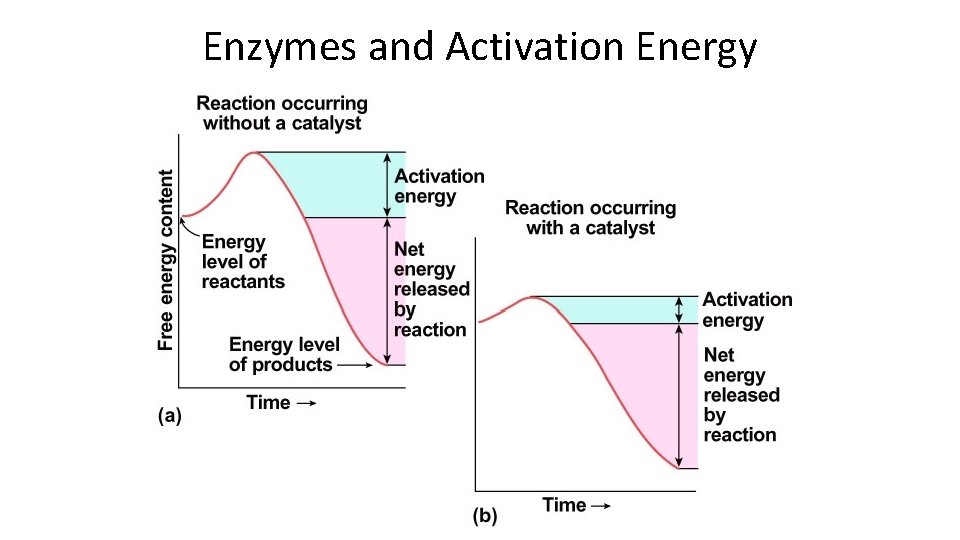
Enzymes and Activation Energy
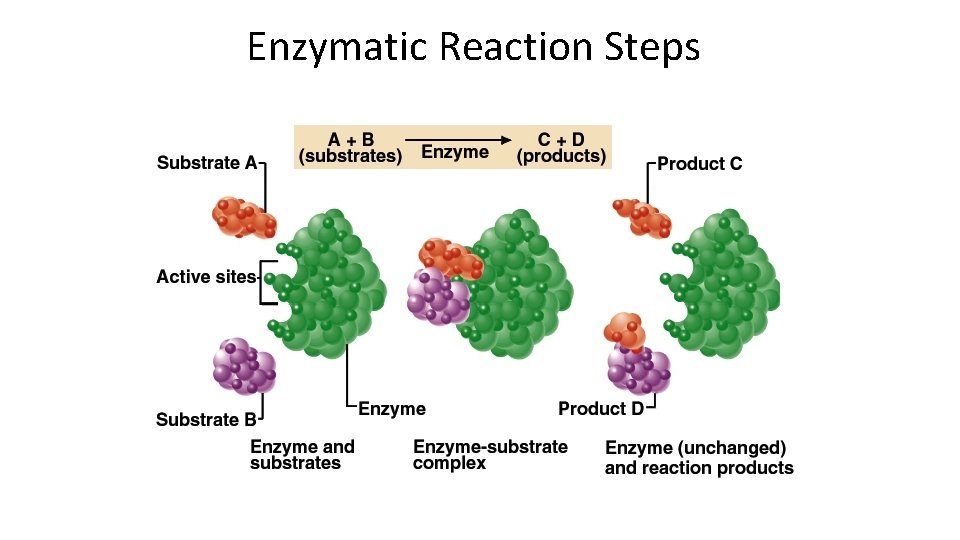
Enzymatic Reaction Steps
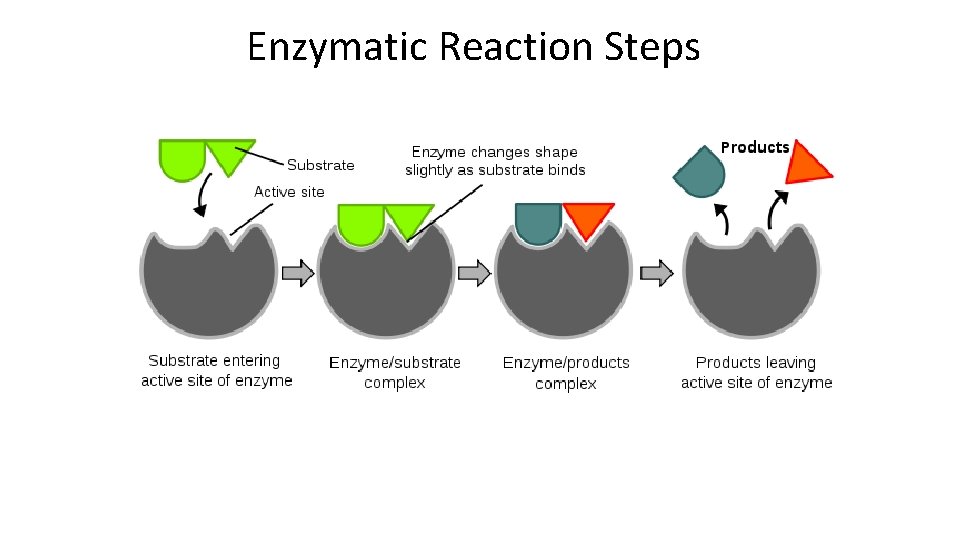
Enzymatic Reaction Steps

PROTEIN Function Structures for movement, structure, protection Catalysis (helping chemical reactions) these proteins are known as enzymes Energy about 4 calories/gram


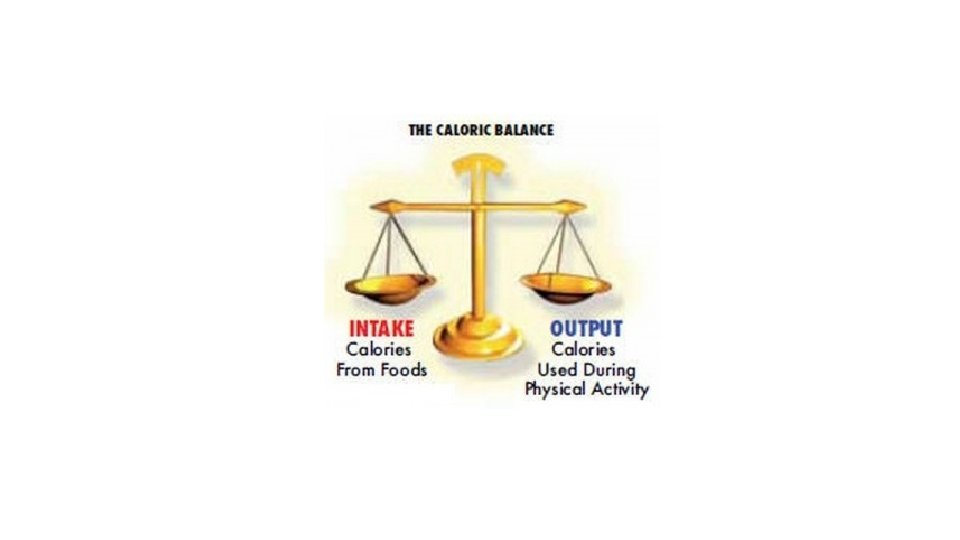
Macronutrients and Energy • What organic molecules can we use for energy? • What is a calorie?



NUCLEIC ACID General Properties Slightly acidic Found in cells and viruses Very long polymer

NUCLEIC ACID Monomer NUCLEOTIDE
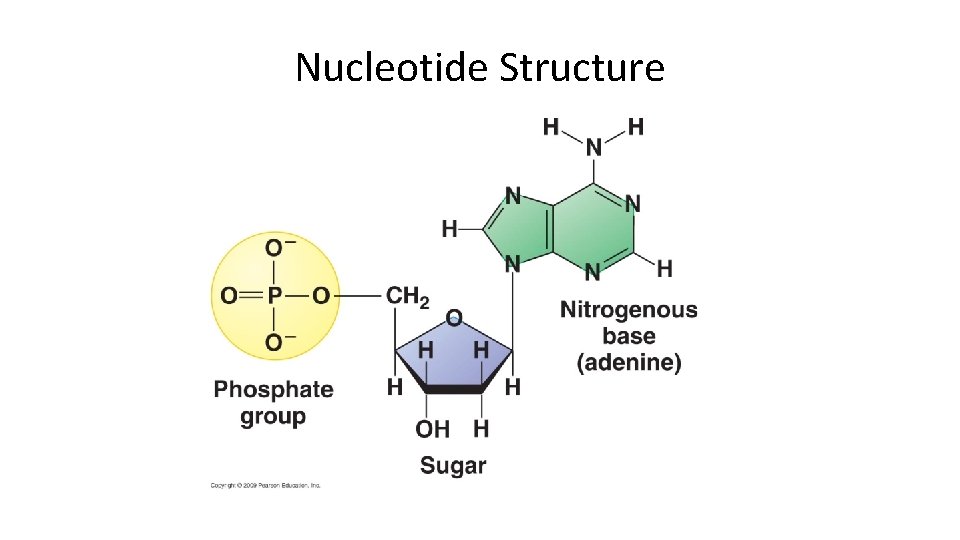
Nucleotide Structure
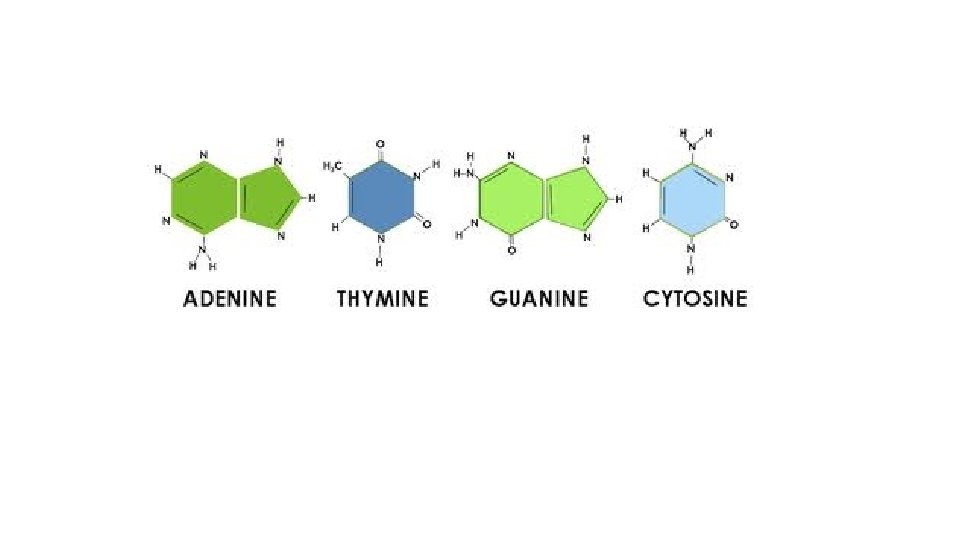

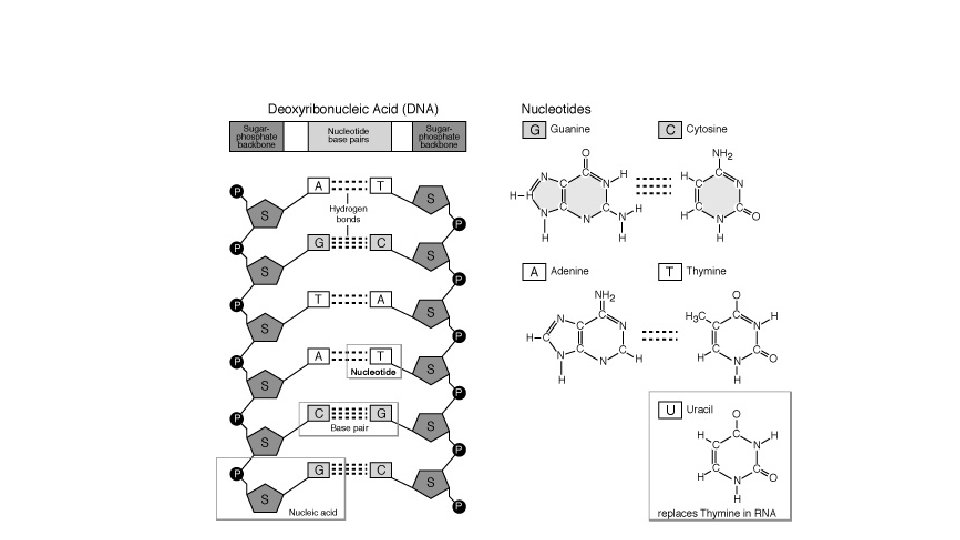
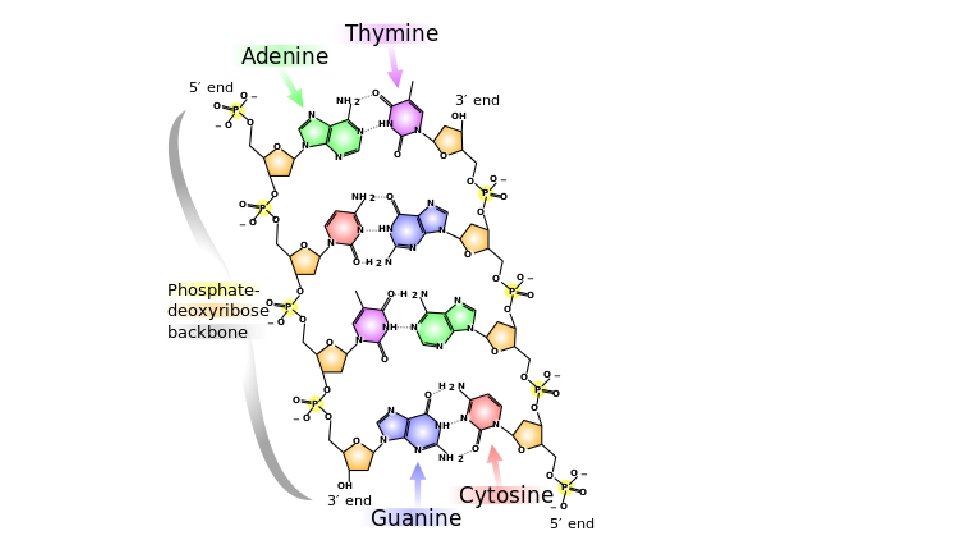
NUCLEIC ACID Polymer NUCLEIC ACID – DNA and RNA
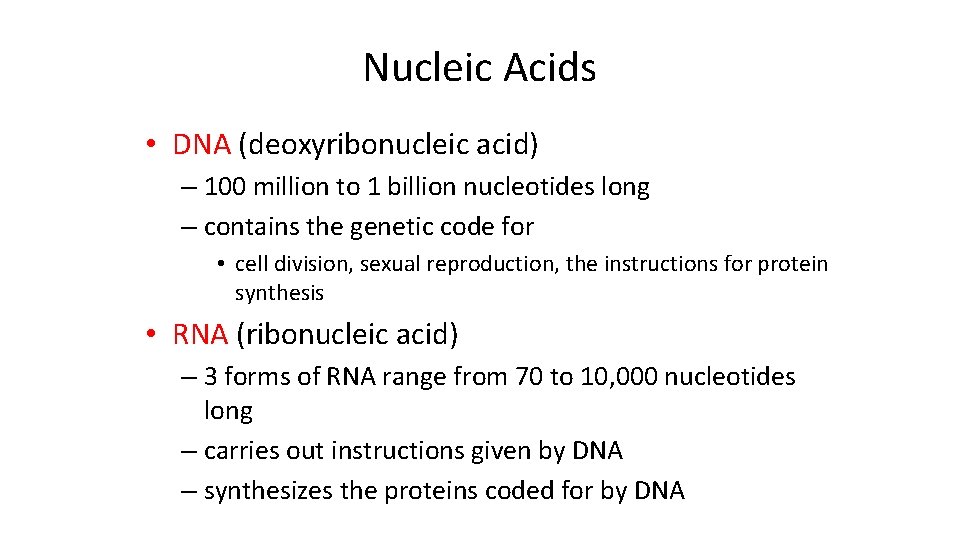
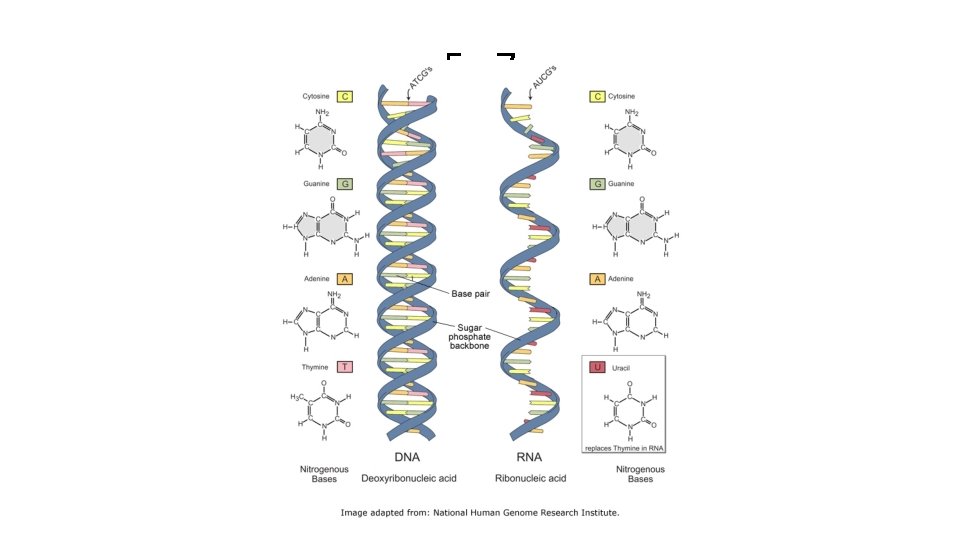
Nucleic Acids • DNA (deoxyribonucleic acid) – 100 million to 1 billion nucleotides long – contains the genetic code for • cell division, sexual reproduction, the instructions for protein synthesis • RNA (ribonucleic acid) – 3 forms of RNA range from 70 to 10, 000 nucleotides long – carries out instructions given by DNA – synthesizes the proteins coded for by DNA
5. x 7
NUCLEIC ACID • Function – store and transmit genetic (hereditary) information by coding for the production of proteins
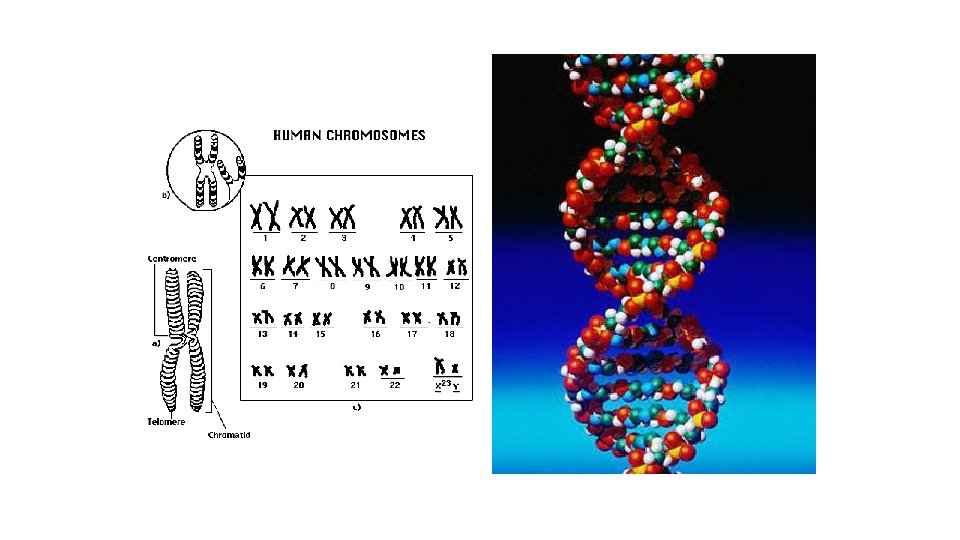
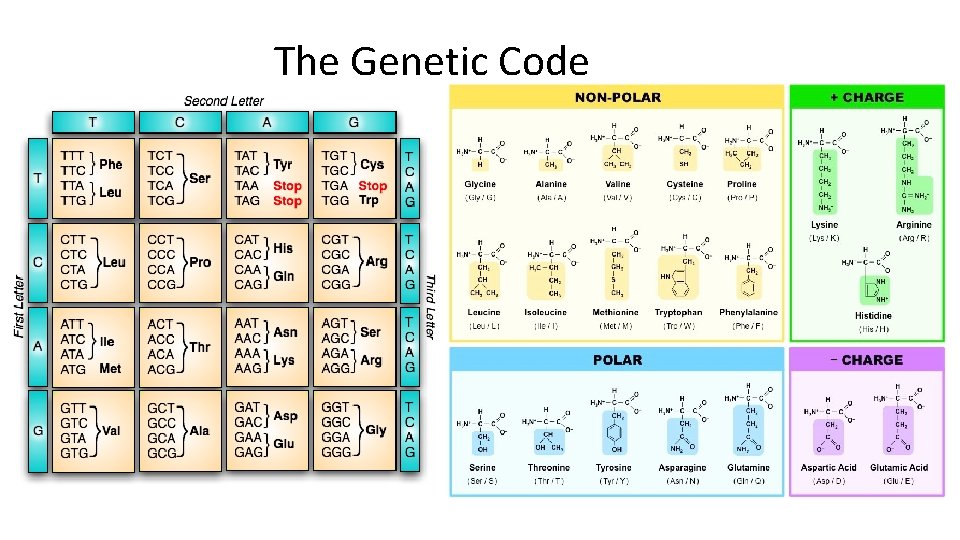
The Genetic Code
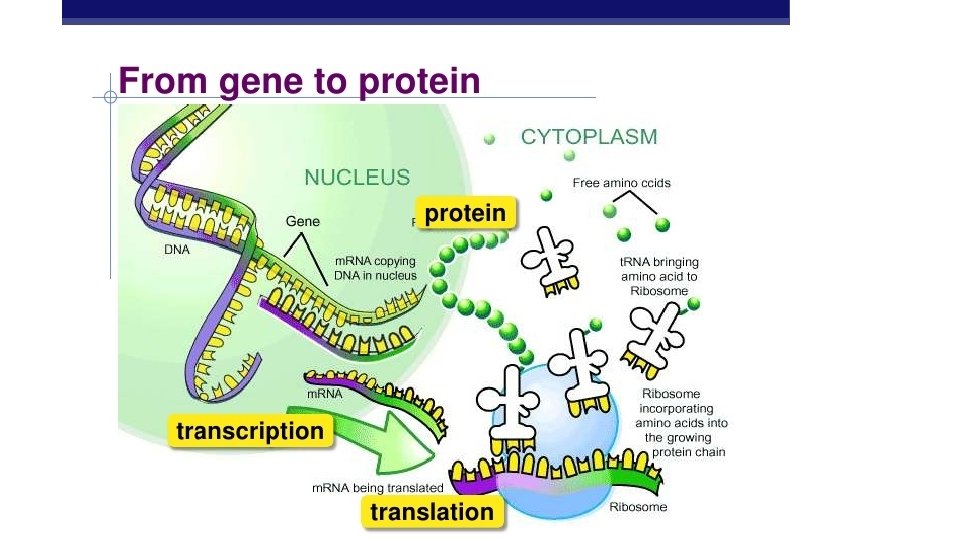
- Slides: 88